Gold sol (reduction)
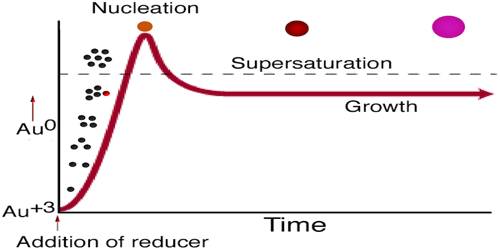
This sol is of particular interest since it is the earliest sol prepared and studied. It was known well before Graham’s definition of colloid. The celebrated purple of cassius was known as early as 1663 A.D. Quite a number of methods are known by which this sol is prepared. Gold Sol is Colloidal Gold. It can be produced by boiling a solution of tetracholoroauric acid with a reducing agent. At the beginning of the reduction process, the gold atoms are liberated from the chloroauric acid.
All of these methods are based on the reduction of a gold compound iii solution by a suitable reducing agent. The reducing agents used are carbon monoxide, hydrogen phosphine, hydrazine, formaldehyde, phosphorus, phenylhydrazine, glucose etc. These reducing agents do not form any strong electrolyte and consequently, dialysis is not required. If, however, stannous chloride is used as the reducing agent dialysis is necessary.
About 10-15 mg of gold chloride is dissolved in about 100 mL of clean water. To this is added about 30-40 mg of potassium carbonate. A dilute ethereal solution of phosphorus is made and about 0.5 mL of it is mixed with the gold chloride solution. A bright red gold sol is then produced by the reduction of the chloride. A similar gold sol can be prepared using formaldehyde as the reducing agent, other steps being nearly identical.
The formation of uniform gold sols produced by the citrate reduction of auric acid is explored as a function of temperature and reagent concentration. As aurate ions are reduced, the reaction medium changes from black to purple to blue before turning deep red. These color changes are shown to result from a decrease in particle size over the course of the reaction.