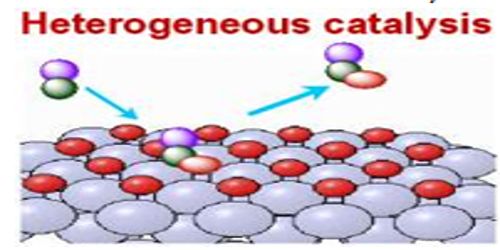
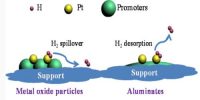
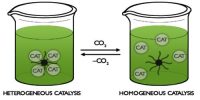
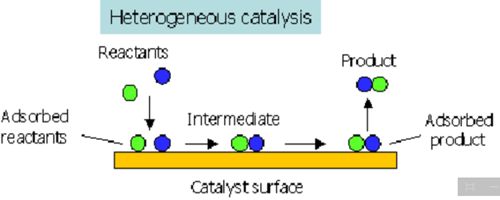
Heterogeneous catalysis
Heterogeneous catalysis a process where you have one material supporting the actual catalyst for a reaction. If in a catalyzed reaction the catalyst itself is in a different phase from the reactants the phenomenon is called heterogeneous catalysis. In heterogeneous catalysis the reactions take place at the interface of two phases. The catalyst is, often a solid and adsorbs a liquid or a gas. This type of catalysis is of great importance in many industrial processes. Examples are:
(i) Manufacture of ammonia by the Haber process. Iron (Fe) acts as catalyst.
N2 (g) + 3H3 (g) → 2NH3 (g)
(ii) Manufacture of sulphuric acid by the Contact process. Vanadium pentoxide (V2O5) or platinum are catalysts for the production of SO3 (g) from SO2 (g) and O2 (g).
2SO2 (g) + O2 (g) → 2SO3 (g)
(iii) Nickel or platinum as catalyst for the hydrogenation of oils and fats.
(iv) Catalysts used in many reactions in the petroleum and polymer industries. There are cases of heterogeneous catalysis where a reaction in the liquid phase is catalyzed by a substance in the solid state. An example is the decomposition of H2O2 (aqueous) by MnO2 (s).
2H2O2 (aq) → 2 H2O (l) + O2 (g)
Again there are examples of reactions in which both the reactant and the catalyst are in the solid phase. The decomposition of KClO3 is catalyzed by solid MnO2.
2 KClO3 (s) → 2KCl (s) + 3O2 (g)