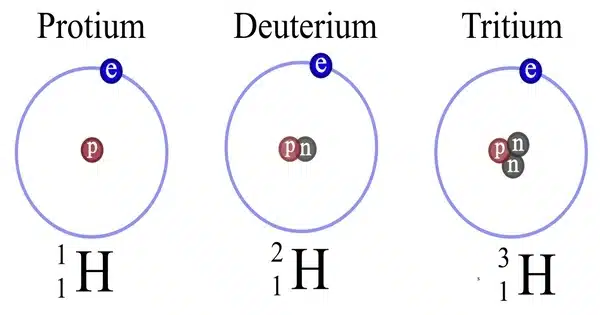
Hydrogen deuteride is a diatomic molecule substance or compound composed of two hydrogen isotopes: 1H (protium) and 2H (deuterium). It is a chemical compound made up of the elements hydrogen and deuterium. Its correct molecular formula is H2H, but for convenience, it is usually written as HD. Deuterium is a stable hydrogen isotope that has a neutron in addition to the proton in the nucleus. HD is made up of one hydrogen atom (H) and one deuterium atom (D) that are held together by a covalent bond.
Hydrogen deuteride has the molecular formula HD. Its structure is similar to that of the more common hydrogen molecule (H2), but one of the hydrogen atoms has been replaced by a deuterium atom. HD is a diatomic molecule, which means it has two atoms.
Preparation and occurrence
In the laboratory it is produced by treating sodium hydride with deuterated water:
NaH + D2O → HD + NaOD
Hydrogen deuteride is a trace element found in naturally occurring molecular hydrogen. It is a minor but noticeable component of the atmospheres of all the giant planets, with abundances ranging from 30 to 200 ppm. HD has also been discovered in supernova remnants and other locations.
Properties
The physical and chemical properties of hydrogen deuteride are similar to those of hydrogen gas, with some differences due to the presence of deuterium. Because of the increased mass of the deuterium atom, HD has a slightly higher boiling and melting point than H2. It also has various spectroscopic properties that can be used for detection and analysis.
- Chemical formula: HD
- Molar mass: 3.02204 g mol−1
- Melting point: −259 °C (−434.2 °F; 14.1 K)
- Boiling point: −253 °C (−423.4 °F; 20.1 K)
Radio emission spectra
HD and H2 have very similar emission spectra, but the emission frequencies differ.
The frequency of the astronomically important J = 1-0 rotational transition of HD at 2.7 THz has been measured with tunable FIR radiation with an accuracy of 150 kHz.
Applications
Hydrogen deuteride is used in a variety of scientific and industrial processes. It is used as a tracer in chemical reactions, particularly in hydrogen bonding research. Due to the properties of deuterium, HD can also be used as a neutron moderator in nuclear reactors. It has also been used as a precursor in the synthesis of deuterated organic compounds for use in pharmaceutical and biomedical research.