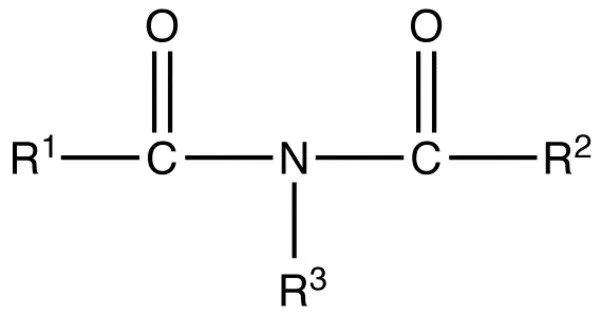
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. These are a class of chemical compounds that contain the imide functional group (-C(=O)NR2), where R is typically a hydrogen atom or an alkyl or aryl group. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−).
Imides can be considered as derivatives of carboxylic acids, where the hydroxyl group (-OH) of the carboxyl group (-COOH) has been replaced by an amino group (-NH2) or an organic group attached to nitrogen (-NR2).
In addition to solid state imides, molecular imides are also known in dilute gases, where their spectrum can be studied. When covalently bound to a metal, an imide ligand produces a transition metal imido complex. When the hydrogen of the imide group is substituted by an organic group, an organoimide results. Complexes of actinide and rare earth elements with organoimides are known.
Properties
Lithium imide undergoes a phase transition at 87 °C where it goes from an ordered to a more symmetric disordered state. The melting and boiling points can also vary significantly. Generally, imides tend to have higher melting and boiling points compared to similarly sized organic compounds due to the presence of polar functional groups. It can display both acidic and basic properties. The nitrogen atom in the imide group can act as a weak base, and the hydrogen atoms attached to nitrogen can be acidic, especially if the substituents are electron-withdrawing.
Structure
Many imides have a cubic rock salt structure, with the metal and nitrogen occupying the main positions. The position of the hydrogen atom is hard to determine, but is disordered. Many of the heavy metal simple imide molecules are linear. This is due to the filled 2p orbital of nitrogen donating electrons to an empty d orbital on the metal.
Formation
- Heating lithium amide with lithium hydride yields lithium imide and hydrogen gas. This reaction takes place as released ammonia reacts with lithium hydride.
- Heating magnesium amide to about 400 °C yields magnesium imide with the loss of ammonia. Magnesium imide itself decomposes if heated between 455 and 490 °C.
- Beryllium imide forms from beryllium amide when heated to 230 °C in a vacuum.
- When strontium metal is heated with ammonia at 750 °C, the dark yellow strontium imide forms.
- When barium vapour is heated with ammonia in an electrical discharge, the gaseous, molecular BaNH is formed. Molecules ScNH, YNH, and LaNH are also known.
Classification
Inorganic imides can be further classified based on the metal or non-metal element that is bonded to the imide functional group. Some common examples of inorganic imides include:
- Metal Imides: These are compounds in which the imide group is bonded to a metal atom. One well-known example is lithium imide (Li2NH), which is used as a hydrogen storage material in some advanced energy storage systems.
- Alkali Metal Imides: These are imides formed by alkali metals (e.g., lithium, sodium, potassium) with ammonia or amines. They are often used as reagents in organic and inorganic synthesis.
- Aluminum Imides: These are compounds where aluminum is bonded to the imide group. An example is aluminum imide chloride (AlNCl), which is used in the synthesis of various organic compounds.
Applicatiuons
Inorganic imides can have various applications in catalysis, materials science, and chemical synthesis. They often serve as intermediates in the preparation of more complex compounds and are important in the study of coordination chemistry and organometallic chemistry. Additionally, some inorganic imides have been investigated for their potential use in energy storage and conversion technologies due to their unique properties.