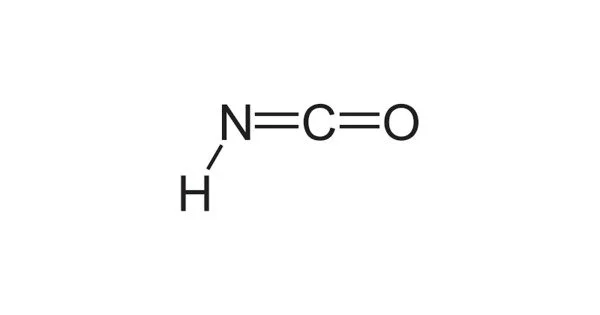
Isocyanic acid is a chemical molecule having the molecular formula HNCO, often known as H−N=C=O. It has a boiling point of 23.5 °C and is colorless, volatile, and toxic. It has a strong stench and is extremely poisonous. It is the most common tautomer and isomer of cyanic acid (aka cyanol) (H−O−C≡N).
Isocyanic acid is an isomer of cyanic acid (HCNO) with a different atom configuration. Isocyanic acid is made up of a nitrogen atom (N) that is single-bonded to a carbon atom (C) and an oxygen atom (O). H-N=C=O is the chemical formula for this compound.
The derived anion of isocyanic acid is the same as the derived anion of cyanic acid, and that anion is [N=C=O]−, which is called cyanate. The related functional group −N=C=O is isocyanate; it is distinct from cyanate (−O−C≡N), fulminate (−O−N+≡C−), and nitrile oxide (−C≡N+−O−).
Properties
- Chemical formula: HNCO
- Molar mass: 43.025 g·mol−1
- Appearance: Colorless liquid or gas (boiling point near room temperature)
- Density: 1.14 g/cm3 (20 °C)
- Melting point: −86 °C (−123 °F; 187 K)
- Boiling point: 23.5 °C (74.3 °F; 296.6 K)
- Solubility in water: Dissolves
- Solubility: Soluble in benzene, toluene, diethyl ether
- Conjugate acid: Oxomethaniminium
- Conjugate base: Cyanate
Isocyanic acid was discovered in 1830 by Justus von Liebig and Friedrich Wöhler.
Isocyanic acid is the simplest stable chemical compound that contains carbon, hydrogen, nitrogen, and oxygen, the four most commonly found elements in organic chemistry and biology. It is the only fairly stable one of the four linear isomers with molecular formula HOCN that have been synthesized, the others being cyanic acid (cyanol, H−O−C≡N) and the elusive fulminic acid (H−C≡N+−O−) and isofulminic acid H−O−N+≡C−.
Application
Isocyanic acid is employed in a variety of chemical reactions and industrial processes, most notably in the synthesis of urethanes and isocyanates. These compounds are used in the production of plastics, foams, and other materials.
Safety
Because of their toxicity and reactivity, isocyanic acid and its derivatives should be handled with caution because they can be dangerous to human health. When working with these compounds, proper safety precautions and protective equipment are required.