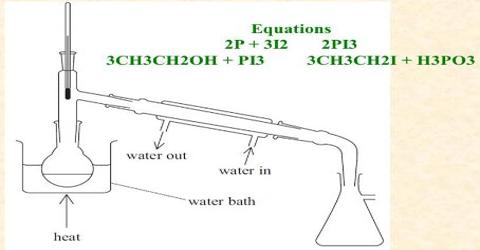
Laboratory Preparation of Iodomethane:
In laboratory, iodomethane is prepared by the reaction of methanol, red phosphorus & Iodine. The reaction occurs in two steps- at first, phosphorus tri-iodide is formed by the reaction of phosphorus & iodine. Phosphorus tri-iodide then reacts with methanol forming iodomethane (methyl iodide).
2P + 3I2 → 2PI3
PI3 + 3CH3 – OH → 3CH3 – I – H3PO3
Procedure: At first methyl alcohol and red phosphorus are taken in a round bottomed flask and iodine is added to it in little amount as shown in Figure. While adding iodine, phosphorus tri-iodide (PI3) produced, create much heat. That’s why; the round bottom flask is dipped in cold water often, to make it cool down. In the 2nd step methyl alcohol reacts with PL3 to form iodo-methane and phosphorus acid. After keeping the reacting mixture for several hours at rest, the iodo-methane is separated from the mixture by common distillation and it is collected in a receiver placed in a water bath.
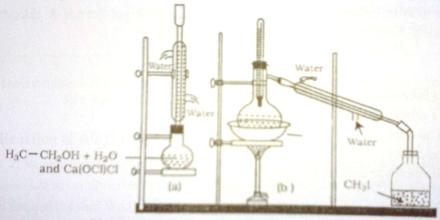
Fig: Laboratory preparation of iodo-methane
(a) Preparation of CH3I (b) Collection of CH3I by distillation
Purification: Some impurities like HI, I2 and CH3OH remain in the collected CH3I. If the mixture is added in caustic soda and stirred thoroughly then H2 and I2 will react with caustic soda forming NaI which will dissolve in the aqueous layer. The organic layer of CH3I remains separated from that aqueous layer of NaI. CH3I is then separated by a separating funnel. Now when CH3I is refined with CaCl2 (boiling point CH3I is 42.3O C) we get purr and dry CH3I.