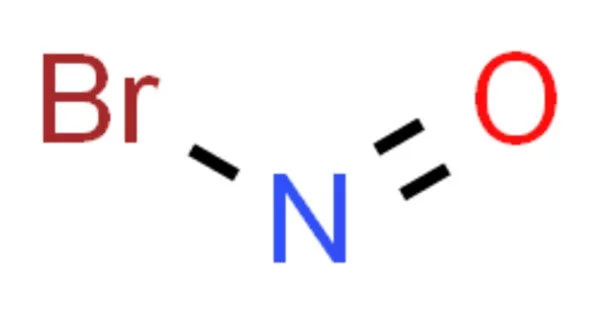
The chemical compound with the formula NOBr is nitrosyl bromide. It is a red gas with a condensing point that is slightly lower than room temperature. At temperatures between -7° and -15° C, the direct union of nitric oxide with bromine occurs when the gas is led into bromine until no further absorption occurs. The additive compound NOBr.Br2 is formed when the temperature rises above -7° C.
The reversible reaction of nitric oxide with bromine can produce nitrosyl bromide. This reaction is intriguing because it is one of only a few third-order homogeneous gas reactions. At standard pressure and temperature, NOBr is susceptible to photodisassociation.
2 NO + Br2 ⇌ 2 NOBr
Properties
The chemical compound NOCl is nitrosyl chloride. It is a yellow gas that is most commonly encountered as a byproduct of the decomposition of aqua regia, a mixture of hydrochloric acid and nitric acid. The related nitrosyl halides nitrosyl fluoride, NOF, and nitrosyl bromide, NOBr, are also known.
- Chemical formula: NOBr
- Molar mass: 109.910 g/mol
- Appearance: Red gas
- Boiling point: 14.5 °C (58.1 °F; 287.6 K)
Nitrosyl bromide is a brownish-black liquid that dissociates at -2° C. In general, nitrosyl bromide and nitrosyl chloride have very similar properties.
Nitrosyl bromide is formed at a temperature of between 9.000 and 10.500 degrees Celsius. The fusion curve of bromine and nitric oxide exhibits a eutectic at – 40° C., corresponding to NOBr3 or NOBr.Br3, and another at -55° C., corresponding to NOBr2 and NOBr.
The compound NOBr3 is a brownish opaque liquid that decomposes at 32° C. At 20° C., the density is 2.637. At 22° C., the heat of formation is 27.000 Cals. A wide range of organic compounds react with nitryl fluoride.