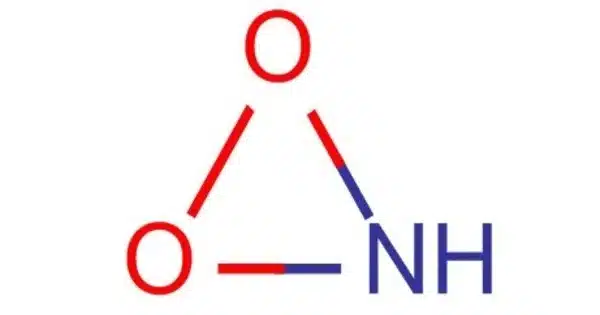
Nitrosyl-O-hydroxide (molecular formula HOON) is an isomer of nitrous acid that has been found experimentally in the gas phase. It’s a chemical molecule with the molecular formula HNO2. HOON has the longest oxygen-oxygen bond in any known molecule, measuring 1.9149 angstroms. It is, in fact, an isomer of nitrous acid (HNO2) with a distinct atomic structure. There had been discussion regarding the existence of this molecule, and ab initio calculations revealed that it could have a stable chemically linked structure.
Properties
- Molecular Weight: The molecular weight is approximately 63.01 g/mol.
- Structure: The compound has a linear structure with a nitrogen atom bonded to an oxygen atom (nitrosyl group) and both of these atoms are bonded to a single oxygen atom with a hydroxyl group.
- Stability: It is not stable and is prone to decomposition, especially in the presence of heat, light, or other reactive chemicals. It can decompose into various nitrogen oxides and water.
- Reactivity: It is a reactive compound and can participate in chemical reactions, especially those involving nitrogen oxides or oxidizing agents.
- Appearance: It is a colorless or pale yellow liquid at room temperature and pressure.
- Solubility: It is miscible in water and many organic solvents, making it relatively easy to handle in laboratory settings.
Nitrous acid (HNO2) is made up of a nitrogen (N) atom coupled to two oxygen (O) atoms, one of which is bonded to a hydrogen (H) atom. The chemical formula for it is H-O-N=O.
Nitrosyl-O-hydroxide (HNO2), on the other hand, is made up of a nitrogen (N) atom connected to an oxygen (O) atom, which is bonded to a hydrogen (H) atom and another oxygen (O) atom. The chemical formula for it is H-N=O-O. While both molecules include nitrogen, oxygen, and hydrogen atoms, their structural configurations differ, making them isomers of one another. Because of their diverse structures, these isomers can have varying chemical characteristics and reactivities.
Applications
Nitrosyl-O-hydroxide does not have significant practical applications, but it may be used as a reagent in certain laboratory reactions involving the generation of nitrosyl groups.
Safety
Due to its reactivity and potential for decomposition, nitrosyl-O-hydroxide should be handled with care. It is not commonly used in practical applications and is primarily of interest in chemical research.