One Component Phase Systems: Sulphur System
The phase diagram of sulphur is somewhat more complicated than that of water although both are one component systems. A phase is defined as “a homogeneous, physically distinct and mechanically separable portion of the system, which is separated from other such parts of the system by definite boundary surface”.
Sulphur can exist in four different phases:
(i) Two well-defined crystalline allotropic forms in the solid state, e.g.,
- Monoclinic sulphur, Sm
- Rhombic sulphur, Sr
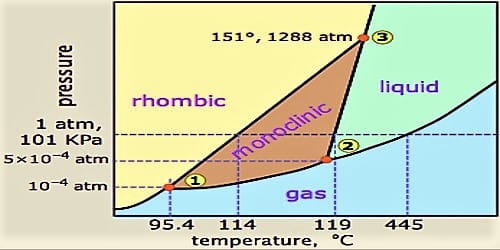
At room temperature rhombic sulphur is more stable and on heating, it passes on to monoclinic sulphur which exists within a range of temperature and pressure hounded by PQR (Figure). The temperature at which rhombic sulphur passes on to the monoclinic form is 95.50 C. This is known as the transition temperature for rhombic sulphur to get converted into monoclinic sulphur.
(ii) Liquid Sulphur, Sl.
(iii) Sulphur Vapour, Sv
In all these phases we are dealing with the same chemical entity, sulphur. So, it is a one component system. The system can be easily understood if the water system has been carefully studied. The important features of this system will be clear if reference is made to Figure, which is a pressure-temperature (P – t) diagram.
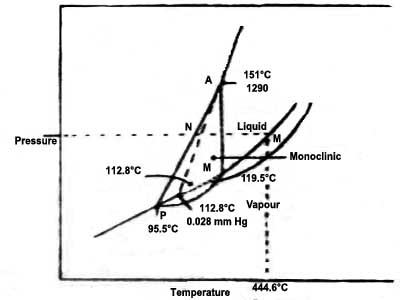
Fig: Phase diagram (P–t) of sulphur (schematic)
In the Figure, the following can be identified:
(a) The solid curves AP, PR, PQ, QB, QR and RT and the dotted curves PS, QS and RS. Alone these curves the system is univariant, i.e., any one of the two variables temperature and pressure will define the system.
(b) Well-defined areas to the right of lines AP, PQ and QB, area above RQB, area to the left of APRT, an area enclosed by PQR. Within any of these areas, the system is bivariant, i.e., the values of both temperature and pressure have to be stated it defines a point.
(c) The points P, Q, R and S. All four of these are triple points as at each point three phases co-exist and the system becomes invariant. The three phases that co-exist are different at different temperatures and pressures.
As in the case of water each of the solid curves shows equilibrium between two phases:
Along AP solid rhombic sulphur is in equilibrium with vapour and the curve shows, the variation of the vapour pressure of rhombic sulphur with temperature. This is the sublimation curve of rhombic sulphur.
Along PR rhombic sulphur is in equilibrium with monoclinic sulphur and the curve shows the variation of the transition temperature for the conversion of rhombic sulphur to monoclinic sulphur at different pressures.
The curve PQ shows the variation of the vapour pressure of monoclinic sulphur with temperature. Along PQ monoclinic sulphur is in equilibrium with vapour. It is the sublimation curve for monoclinic sulphur.
The curve QB shows the variation of the vapour pressure of liquid sulphur with temperature. Along with QB liquid, sulphur and vapour are in equilibrium. The curve shows the effect of pressure on the boiling point at sulphur.
Along with QR monoclinic sulphur ad liquid sulphur is in equilibrium and the curve shows the variation of the melting temperature of monoclinic sulphur with pressure.
The dotted curves PS, QS and RS show temperature/pressure values for the metastable equilibria – PS between rhombic sulphur and vapour. QS between solid monoclinic sulphur and vapour and RS between solid rhombic sulphur and liquid.
The curve RT represents equilibrium between solid rhombic sulphur and liquid sulphur and shows the effect of pressure on the melting point of rhombic sulphur. Similarly, it can be pointed out that to the left of the curve APR the system consists of only rhombic, sulphur, in the area to the right of APQB sulphur exists only in the vapour phase, the area bounded by PQR defines the region of temperature and pressure where monoclinic sulphur can exist and the region between the lines RQ and QB is where sulphur is in the liquid state.