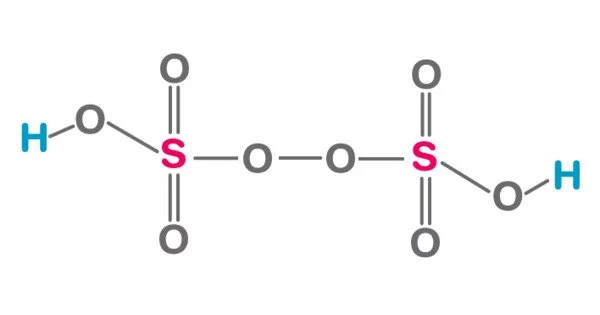
Peroxydisulfuric acid is an inorganic compound with a chemical formula (HO3SO)2. It is a powerful and highly reactive oxidizing agent that contains two peroxy (O-O) groups. It is a derivative of sulfuric acid (H2SO4) in which one oxygen atom is replaced with a peroxy group (-O-O-). Also called Marshall’s acid after Professor Hugh Marshall, who discovered it in 1891.
It is a strong oxidizing agent and can react vigorously with various reducing agents. It is usually a colorless, crystalline solid. It can also be found as a colorless solution in water. It is soluble in water and forms a stable solution known as “Piranha solution” when mixed with sulfuric acid.
Properties
Peroxydisulfuric acid is stable in concentrated sulfuric acid solutions. However, it is highly sensitive to contamination and may decompose explosively when exposed to organic materials, such as paper, cloth, or wood.
- Chemical formula: H2O8S2
- Molar mass: 194.13 g·mol−1
- Appearance: Colourless solid
- Melting point: 65 °C (149 °F; 338 K) (decomposes)
- Solubility in water: soluble
- Conjugate base: Peroxydisulfate
Structure and bonding
This oxoacid features sulfur in its +6 oxidation state and a peroxide group. Sulfur adopts the usual tetrahedral geometry.
Preparation
Peroxydisulfuric acid is typically prepared by mixing concentrated sulfuric acid (H2SO4) with hydrogen peroxide (H2O2). The resulting solution contains peroxydisulfuric acid and other species, and it is often used as a source of active oxygen in various chemical reactions.
Peroxydisulfuric acid is a strong oxidizing agent and can be used in various chemical reactions, including the oxidation of organic compounds. It is often used in laboratories and industrial settings for its ability to introduce oxygen into chemical compounds. However, it is a highly reactive and potentially dangerous chemical, so it should be handled with care, and safety precautions should be taken when working with it.
Uses
Peroxydisulfuric acid is the starting point for many salts such as sodium peroxydisulfate, potassium peroxydisulfate, and ammonium peroxydisulfate. These salts are used to start the polymerization of acrylonitrile, styrene, and other similar monomers. This application takes advantage of the peroxydisulfate anion’s proclivity to undergo hemolysis and generate radicals. They’re also used to clean circuit boards.
Safety Precautions
Due to its strong oxidizing properties, Peroxydisulfuric acid is extremely hazardous and should be handled with great care. It can cause severe chemical burns and should be used in a fume hood with appropriate protective equipment.