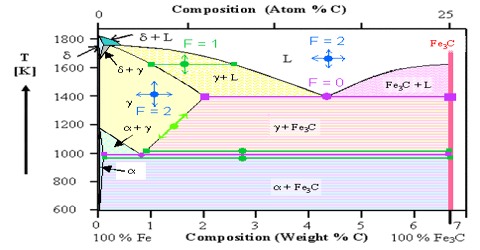
A phase may be defined as: The homogeneous part of a heterogeneous system which is physically and chemically different front other parts of the same system and bounded by surfaces of separation and mechanically separable.
A system can consist of one or more phases. Some examples are given below:
One phase systems:
(a) A pure substance in one physical state.
(b) A mixture of liquids which are completely miscible with each other in all proportions is one phase in the liquid state, e.g., H2O/Alcohol etc.
(c) A pure gas or a mixture of gases which do not react with each other.
(d) A solution of a solid in a liquid, NaCl/H2O, Sugar/H2O etc.
(e) A mixture of solids in the molten state.
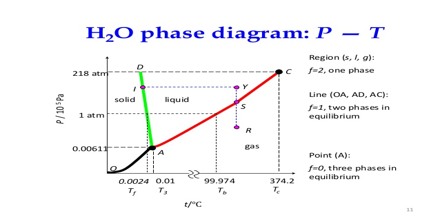
Two phase systems:
(a) A pair of immiscible liquids, e.g., CCl4/H2O, C2H2Cl2/H2O etc.
(b) A liquid and its vapour.
(c) A solid substance and its liquid phase or vapour phase.
(d) A mixture of solids in the liquid (molten) phase and one of the solids in the solid phase.
(e) A mixture of two solids which do not react with each other.
Three phase systems:
(a) A pure substance existing in three phases together, e.g., ice, water and water vapour.
(b) A solid in two allotropic forms and the liquid mixture or in vapour phase.
(c) A mixture of two solids in the molten form and the two solids.