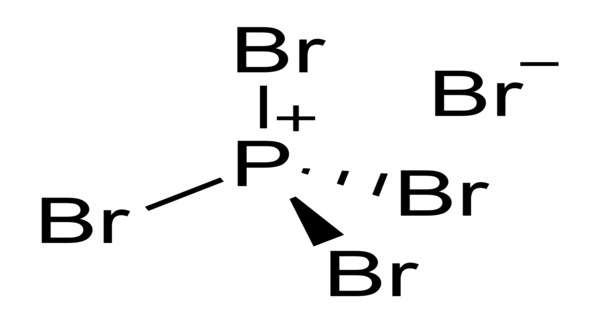
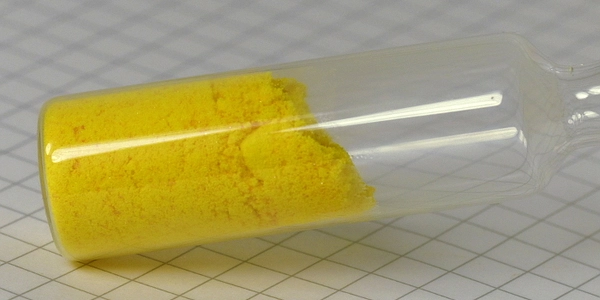
Phosphorus pentachloride is a greenish-yellow crystalline solid that decomposes in water to form hydrochloric acid and phosphoric acid with the release of heat energy. It is a reactive yellow solid with the formula PBr5, which has the solid-state structure PBr+5 Br– but is completely dissociated to PBr3 and Br2 in the vapor phase. It is known to have a salt-like structure in the crystalline state and to be partially dissociated in solution, particularly in polar solvents like nitrobenzene. It is made by reacting dry chlorine with phosphorus trichloride.
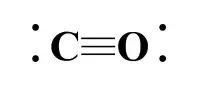
In the solid state, PCl5 prefers to exist as oppositely charged ions such as [PCl4]+ and [PCl6] because ionic bonding improves crystalline nature. In the ionic form, the shape is more stable because it forms the tetrahedral and octahedral shapes.
In organic chemistry, it can be used to convert carboxylic acids to acyl bromides. It is extremely corrosive. Above 100 °C, it decomposes to give phosphorus tribromide and bromine:
PBr5 → PBr3 + Br2
In the presence of phosphorus trichloride or chlorine gas, phosphorus pentachloride vaporizes without dissociation. The presence of the product shifts the dissociation equilibrium to the left. In practice, reversing this equilibrium to produce PBr5 by adding Br2 to PBr3 is difficult because the product is susceptible to further addition to produce phosphorus heptabromide (PBr7).
Properties
- Chemical formula: PBr5
- Molar mass: 430.49 g/mol
- Appearance: yellow solid
- Density: 3.61 g/cm3
- Melting point ca.: 100 °C (decomposes)
- Boiling point: 106 °C (223 °F; 379 K) (decomposes)
- Solubility in water: decomposes
- Solubility: decomposes in ethanol; soluble in CCl4 and CS2
Applications
- Used as a chlorinating agent and catalyst in making organic chemicals, intermediates, dyestuffs, etc.
- Used as a catalyst in the manufacture of acetyl cellulose, the plastic film on which motion pictures are printed.
- In the pharmaceutical industry it is used in the manufacturing of penicillin and cephalosporin.
- Used to produce acid chlorides and as a catalyst for cyclization and condensation reactions.
Harmful Effects
Phosphorus Pentachloride is a chemical that is reactive. Direct Phosphorus Pentachloride exposure can result in fatigue, nausea, headache, dizziness, and vomiting. It has the potential to harm the liver and kidneys. Phosphorus Pentachloride must be identified as a precaution. It is a crystalline solid with a pungent odor and a color ranging from white to pale yellow. Phosphorus Pentachloride is used in the production of other chemicals, aluminum metallurgy, and pharmaceuticals.