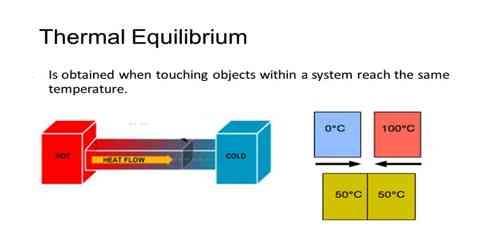
Thermal equilibrium is the circumstance under which two substances in physical contact with each other exchange no heat energy. Two substances in thermal equilibrium are said to be at the similar temperature.
Keep a heated iron ball at a place of room temperature. What will you see? It will be observed that the heated body will continue to lose heat and the process of losing heat will continue unti the temperature of the heated body becomes equal to the temperature of the environment. Similar phenomenon will be observed if two bodies of different temperatures are kept in thermal contact with each other. Then heat is transferred from the body of high temperature to the body at low temperature and at one time both the bodies reach to the same temperature. Then it is said that the two bodies are at thermal equilibrium.
That means, if more than one body is kept in thermal equilibrium and if no heat is exchanged between them it is considered that the bodies are in thermal equilibrium. The thermodynamic law related to this is-
Zeroth law of thermodynamic: If two bodies are in thermal equilibrium with a third body, then the first two bodies must be in thermal equilibrium with each other. It is called the zeroth law of thermodynamics.
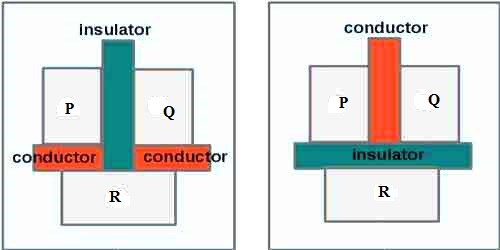
Explanation: In order to determine that the two bodies are in thermal equilibrium, a third body is used. Let P and Q be two bodies separated by a bad-conducting wall are in contact with a third body R (figure). After sometime it will be seen that both P and Q will attain thermal equilibrium in with the third body R. Now, if the bad-conducting wall is taken out, no change of temperature of P and Q will be observed. From this, it is realized that before withdrawing the wall both P and Q reached in thermal equilibrium with each other. From this example the above law is proved. Thermometers are produced on the basis of this law.