Urea: Chemically urea is the di-amine of carbonic acid or carbamide.
Methods of synthesis:
(i) From phosgene gas.
(ii) Commercial process→ from natural gas
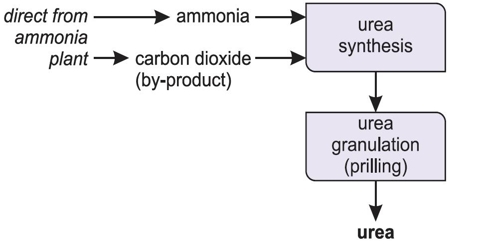
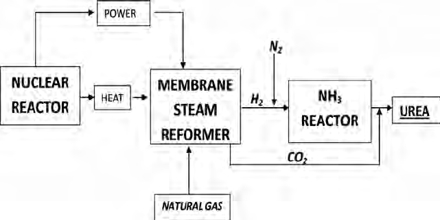
Commercial production of urea
Urea is produced commercially from natural gas. In this process natural gas is combusted to form Carbon dioxide (CO2). Again Ammonia (NH3) is prepared by Haher- Bosch process. Then ammonium carbamate is prepared by the reaction between Carbon dioxide and Ammonia. This is then decomposed by heat to produce urea. A mixture of compressed CO2 and ammonia at 240 barg is reacted to form ammonium carbamate. This is an exothermic reaction, and heat is recovered by a boiler which produces steam. The first reactor acheives 78% conversion of the carbon dioxide to urea and the liquid is then purified.
Use of urea
Urea is the world’s most commonly used nitrogen fertilizer and indeed more urea is manufactured by mass than any other organic chemical.
- Urea is very important fertilizer in agriculture.
- Plastic production: Urea is used to produce urea- formaldehyde resin, which is a polymer containing chains and is broadly used as plastic.