Properties of Radioactive Beta-rays (β-rays)
We know, from radioactive substances three types of rays are emitted. These are α, β, and y-rays. A beta particle also called beta ray or beta radiation, (symbol β) is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. Here Beta-rays (β-rays) properties are described below.
(1) β-rays are extremely light. They are streams of electrons.
(2) The rest mass of β-particle is 9.1 x 10-31 kg.
(3) They carry negative charges. The charge of β-particle is 1.6 x 10-19 C.
(4) β-particles are emitted from different radioactive sources with tremendous speeds varying from 0.9 x 108 ms-1 to 2.9 x 108 ms-1.
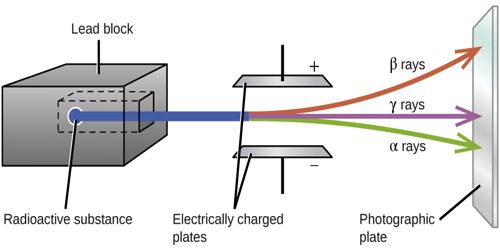
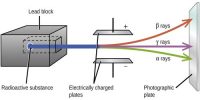
(5) β-rays affect photographic plates. The intensity of the traces produced by β-rays on a photographic plate is greater than the intensity of the traces produced by α-rays.
(6) They ionize gases, but their ionizing power is much less than that of α-particles.
(7) β-rays have penetrating power. Their penetrating power is much greater than that of α-rays.
(8) β-rays cause fluorescence in barium platinocyanide, calcium-tungstate etc.
(9) β-rays are deflected by an electric field.
(10) They are deflected by a magnetic field. From the direction of deflection of these rays by electric and magnetic fields, it is known they carry a negative charge.
(11) β-particles are scattered when they pass through matter. The amount of this scattering is much greater than that of α-rays.
(12) They have kinetic energy.