Radioactive Decay
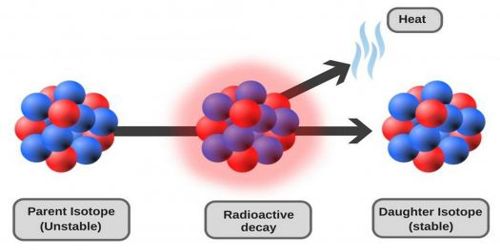
After three years of the discovery of radioactivity scientists Elster and Geitel observed that the radioactivity of a body decreases with time, it is the decay of radioactivity. Decay is said to occur in the parent nucleus and produces a daughter nucleus. It obeys the exponential law. It is the natural breakdown of an atomic nucleus resulting in the release of energy and matter from the nucleus. It is impossible to predict which atoms at a particular moment will disintegrate. It occurs when an unstable atom loses energy by emitting ionizing radiation. The probability of disintegration of an atom of a radioactive substance in unit time is called the decay constant of that substance. The radioactive decay and transmutation process will continue until a new element is formed that has a stable nucleus and is not radioactive. The decay of a radioactive substance obeys the rule of statistics which is known as decay law.
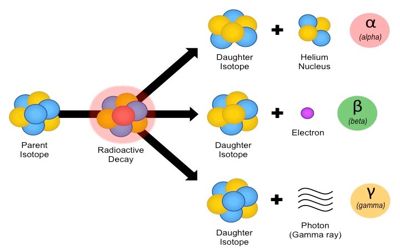
Fig: Radioactive Decay
There are many types of radioactive decay:
Alpha radioactivity. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus.
Beta radioactivity. Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40.
Gamma radioactivity. Gamma radioactivity consists of gamma rays. Gamma rays are electromagnetic radiation of a very high frequency and of a high energy.