Size of colloids
The particles of colloids are not of uniform size. They vary over a wide range. Lyophobic colloids, irrespective of the method of preparation, show often wide divergence in their size — some are small, some are relatively big. Accurate measurement of particle shape and size and drawing up of reliable size distribution cure is still a challenging problem to physical chemists. The complexity of the problem further increases due to different values of particle size of the same colloid obtained by different methods. The molecular colloids are still more complex. They cover a very wide range of molecular mass. It does not matter whether the polymer is natural, like cellulose or rubber, or man-made like polystyrene or nylon. For the same sample in the same solution, the molecular mass range may be from a few thousand to a few hundred thousand. Moreover, for the same solution, the molecular masses determined by different methods may widely differ. Thus all colloids are polydisperse in character.
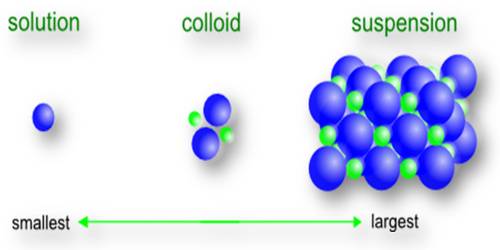
A colloid is typically a two-phase system consisting of a continuous phase (the dispersion medium) and dispersed phase (the particles or emulsion droplets). The particle size of the dispersed phase typically ranges from 1 nanometer to 1 micrometer. Particles of lyophobic colloids undergo quite intense Brownian motion. They move at random in all directions executing zigzag irregular motion. For a short distance interval, the motion is linear. Lyophilic colloids must also hare Brownian motion but this cannot be observed due to their small size. Colloids, therefore, have linear translational motion.