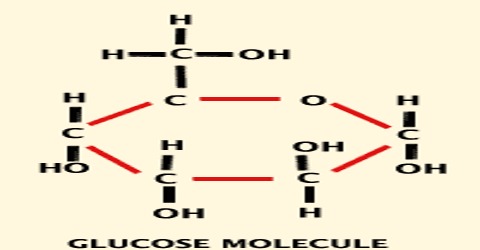
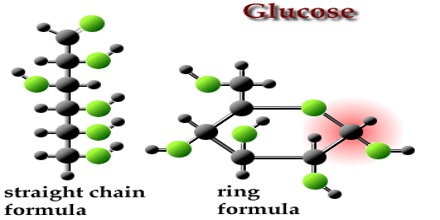
The structure of glucose molecule can be identified by the following reactions:
(i) The structural formula of glucose C6H12O6
(ii) Reduction: Being reduced by HI, glucose forms n-haxane in the presence of red phosphorus. This indicates that, glucose has 6 carbon atoms in its structure which forms a simple chain without branching. So, glucose is a simple chain compound.
(iii) Acetylation: Glucose forms penta-acetate by reacting with acetic anhydrate in the presence of pyridine.
(iv) Oxidation: Being oxidized by bromine water, glucose forms gluconic acid.
(v) Glucose reduces Fehling solution forming red ppt. of Cu2O
(vi) Glucose reduces Tollen’s reagent forming silver mirror.