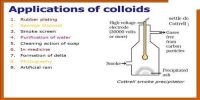
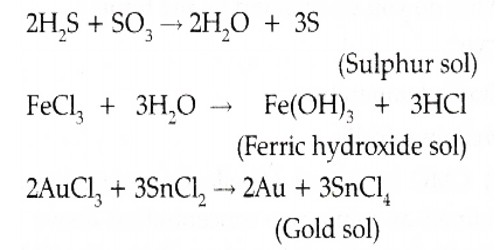Sulphur Sol (oxidation): Preparation
Sulfur sols are colloidal solutions of elemental sulfur or of sulfur-rich compounds. Sulphur sols can be prepared by the oxidation of H2S in solution. The oxidation can be carried out either by oxygen of the air or by a solution of SO2. A Colloidal sol of sulphur can be obtained by passing hydrogen Sulphide into the solution of sulphur dioxide in water or during a solution of an oxidizing agent (Bromine water, nitric acid). There is no need for dialysis since no electrolytes are formed.
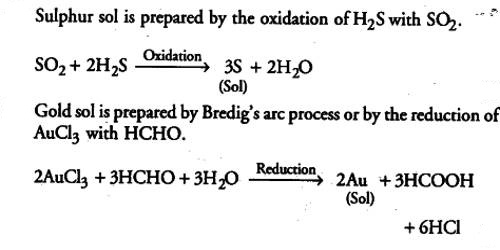
Sol sulphur is prepared by oxidizing an aqueous solution of H2S with bromine water or nitric acid or SO2 or any other appropriate oxidizing agent. Oxidation sulphur sol is prepared by passing H2S into SO2:
H2S + SO2 → S (sol) + H2O
Sulphur sol can also be prepared by the action of a strong acid, say, sulphuric acid, to a concentrated solution of sodium thiosulphate. Sulfur sols are colloidal solutions of elemental sulfur or of sulfur-rich compounds. The particles in these solutions have diameters of 0.1–1.0 μm and consist either of S8 molecules (hydrophobic sulfur sols) or of chain-like sulfur compounds with hydrophilic end groups like sulfonate or functionalized organic groups (hydrophilic sulfur sols). Both types of sols are stabilized by the negative charge of the particles which results in mutual repulsion.
Many sols can be prepared by the similar principle of condensation or agglomeration but without any chemical reaction. Thus if a concentrated solution of a substance is poured into another liquid in which the solubility of the substance is less, it will be thrown out of the solution due to supersaturation and a colloid may result. If an alcoholic solution of phosphorus is poured into water, the phosphorus is thrown out of the solution and under appropriate conditions passes on to colloidal state.
Preparation of Colloidal Sulphur: When H2S in water (aqueous solution) is exposed to air; it gradually gets oxidized to sulphur. The sulphur so produced remains in water in the colloidal state and the solution so produced remains in water in the colloidal state and the solution has a somewhat milkfish emergence.
- H2S + O2 → H2O + 2S (colloidal)
A sol of sulphur can also be arranged when H2S gas is bubbled through an aqueous solution of 5O2.
- H2S + 5O2 → 2 H2O + 3S (colloidal)
Several modifications of the method have been made and these can be used for the preparation of sols. Sols of these types occur both in industrial desulfurization plants where sulfide is oxidized to elemental sulfur as well as in cultures of certain oxidizing sulfur bacteria.