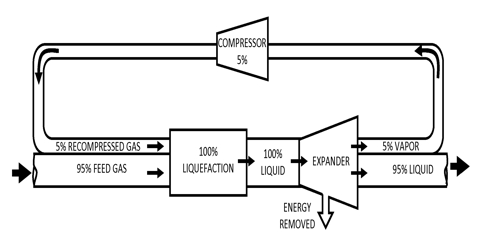
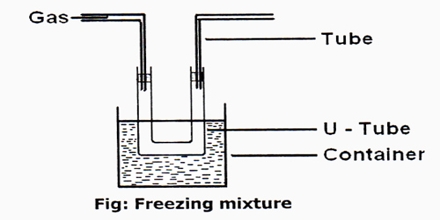
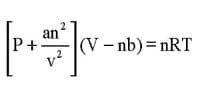Freezing mixtures a mixture (as of salt and ice or of dry ice and acetone) for producing intense cold. Freezing mixtures are useful for producing temperature below 0°C but their use is limited because it is difficult to prepare freezing mixtures which will produce very low temperatures. Solid carbon dioxide and ether could be used to attain a temperature of —110°C.
Evaporating Liquid Gases at Atmospheric Pressure
- Liquid Oxygen : temperature -1830 C
- Liquid Nitrogen : temperature -1960 C
- Liquid Helium : temperature -2690 C
Freezing-point depression describes the process in which adding a solute to a solvent decreases the freezing point of the solvent. Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities in a finely powdered drug.
It a combination of substances that when mixed lower the temperature of the mixture by absorbing the melting heat or the heat of solution of the system’s components, which can be liquid, solid, or both.
Freezing mixtures are used mainly in the laboratory to generate and maintain low temperatures. Industrial requirements for low temperatures are fulfilled by a freezing mixture that consists of crushed ice and reagent grade table salt, or NaCl.