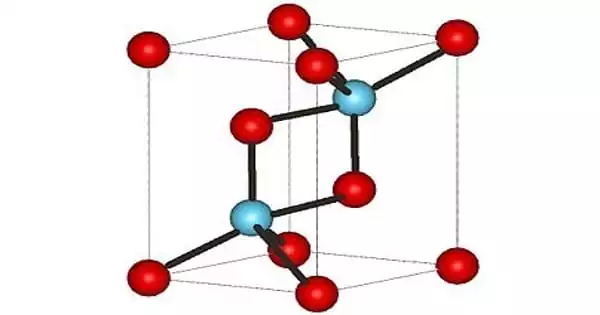
Actinium(III) oxide is a chemical compound containing the uncommon radioactive element actinium. It has the formula Ac2O3. It is identical to its counterpart lanthanum chemical, lanthanum(III) oxide, and contains actinium in the oxidation state +3. Actinium oxide is not to be confused with Ac2O (acetic anhydride), where Ac is an abbreviation for acetyl rather than the element actinium’s symbol.
Actinium is a radioactive element with the atomic number 89 and the element symbol Ac. Although several radioactive elements had been identified prior to actinium, it was the first non-primordial radioactive element to be separated. This element possesses a number of strange and intriguing properties. Here are the properties, uses, and sources of Ac.
Properties
Actinium(III) oxide is a chemical compound containing the radioactive element actiniuim and oxygen. It can be represented as Ac2O3. Actinium is a very rare element and it loses its lustre in air and burns readily to form actinium III oxide. Molar mass of actinium III oxide is 501.9982.
- Category: Organic
- Appearance: White
- Molar Mass / Molecular Weight: 501.998 g/mol
- Boiling Point: None
Because radioactivity ionizes air, actinium is a delicate, silver-colored metal that glows pale blue in the dark. Actinium interacts with moisture and oxygen to generate actinium oxide, a white covering that shields the underlying metal from further oxidation.
Reactions
Ac2O3 + 6HF → 2AcF3 + 3H2O
Ac2O3 + 6HCl → 2AcCl3 + 3H2O
4Ac(NO3)3 → 2 Ac2O3 + 12NO2 + 3O2
4Ac + 3O2 → 2Ac2O3
Ac2O3 + 2AlBr3 → 2AcBr3 + Al2O3
2Ac(OH)3 → Ac2O3 + 3H2O
Ac2(C2O4)3 → Ac2O3 + 3CO2 + 3CO
Ac2O3 + 3H2S → Ac2S3 + 3H2O
Occurrences
Actinium can be found in trace concentrations in uranium and thorium ores. Because actinium is difficult to extract from ore, the most frequent method of production is neutron irradiation of Ra-226. Within nuclear reactors, milligram samples can be prepared in this way.
Health Hazards
Actinium does not appear to have a biological role. It’s radioactive as well as toxic. It is less hazardous than the radioactive elements plutonium and americium. When rats were given actinium trichloride, nearly half of it was deposited in the liver and one-third in the bones. Actinium and related constituents should only be handled with a glove box because to the health risks they pose.