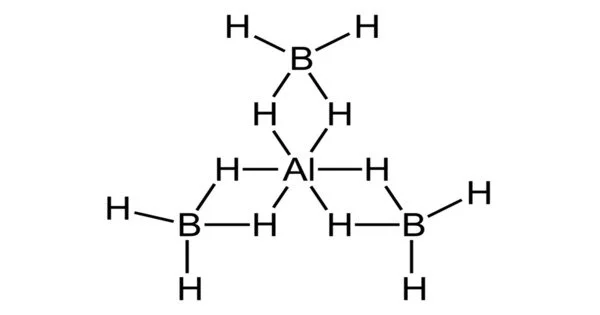
Aluminium Borohydride [Al(BH4)3] is a chemical compound that can be used as a reducing agent, meaning it can donate electrons to other molecules. It is also a hydrogen storage material, as it can release hydrogen gas upon heating or in the presence of a suitable catalyst. Aluminium borohydride is considered to be a promising material for hydrogen storage due to its high hydrogen content, stability, and low toxicity. However, it is not yet widely used in commercial applications due to the high cost and difficulty of synthesizing the material.
It is a clear, colourless liquid. It is slightly denser than water. Contact causes burns to skin, eyes, and mucous membranes. It is toxic by ingestion, inhalation and skin absorption. It is used to make other chemicals.
Properties
Aluminium borohydride is a chemical compound with the formula Al(BH4)3. It is a volatile pyrophoric liquid which is used as a reducing agent in laboratories. Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound.
- Chemical formula: AlB3H12
- Molar mass: 71.51 g·mol−1
- Appearance: colorless liquid
- Melting point: −64.5 °C (−84.1 °F; 208.7 K)
- Boiling point: 44.5 °C (112.1 °F; 317.6 K)
- Solubility in water: reacts
- Flash point: Spontaneously ignites
Preparation
Aluminium borohydride is formed by the reaction between sodium borohydride with aluminium chloride:
3 NaBH4 + AlCl3 → Al(BH4)3 + 3 NaCl
or as the non-pyrophoric tetrahydrofuran (THF) adduct, by the analogous reaction of calcium borohydride and aluminium chloride in THF:
3 Ca(BH4)2 + 2 AlCl3 → 3 CaCl2 + 2 Al(BH4)3
Reactions
Like all borohydrides, this compound is a reducing agent and hydride donor. It reacts with water to give elemental hydrogen gas, and reduces carboxylic esters, aldehydes, and ketones to alcohols.
Application
It is primarily used as a reducing agent and a hydrogen storage material. It can release hydrogen gas when it reacts with water or other protic solvents. It is also used as a fuel for hydrogen fuel cells. Due to its high hydrogen content, it is considered a promising material for hydrogen storage, which is a key component of hydrogen fuel cell technology.