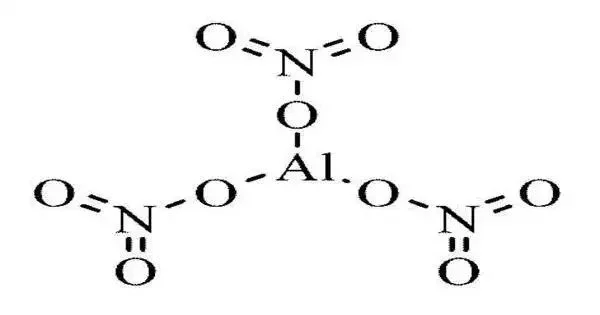
Aluminium nitrate is a white, water-soluble salt of aluminium and nitric acid, most commonly existing as the crystalline hydrate, aluminium nitrate nonahydrate, Al(NO3)3·9H2O. It is a white, crystalline solid that is soluble in water and ethanol. It is a strong oxidizing agent and can react violently with reducing agents. It can also react with alkaline substances to form aluminum hydroxide.
Aluminum nitrate is used in the production of other aluminum compounds, such as aluminum oxide and aluminum hydroxide. It is also used in the manufacture of ceramics and in the treatment of wastewater. In addition, it is used as a catalyst in various chemical reactions.
Properties
It is a white, crystalline solid that is soluble in water and other polar solvents. The molecular weight is 213.00 g/mol. The melting point is 73 °C, and its boiling point is 135 °C. It is highly soluble in water, with a solubility of 880 g/L at 20 °C. It is also soluble in ethanol. It is a strong oxidizing agent and can react violently with reducing agents. It can also react with alkaline substances to form aluminum hydroxide.
- Chemical formula: Al(NO3)3
- Molar mass: 212.996 g/mol (anhydrous); 375.134 g/mol (nonahydrate)
- Appearance: White crystals, solid; hygroscopic
- Odor: odorless
- Density: 1.72 g/cm3 (nonahydrate)
- Melting point: 66 °C (151 °F; 339 K) (anhydrous); 73.9 °C (165.0 °F; 347.0 K) (nonahydrate)
- Boiling point: 150 °C (302 °F; 423 K) (nonahydrate) decomposes
- Solubility in methanol: 14.45 g/100ml
- Solubility in ethanol: 8.63 g/100ml
- Solubility in ethylene glycol: 18.32 g/100ml

Preparation
Aluminium nitrate can be prepared by reacting aluminium metal or aluminium hydroxide with nitric acid. It has a molar mass of 212.996 g/mol and a density of 1.72 g/cm³. Its melting point is 73 °C, and it decomposes at 150-180 °C.
Aluminium nitrate cannot be produced by reacting aluminium with concentrated nitric acid because the aluminium forms a passivation layer. It can also be made by reacting nitric acid with aluminium(III) chloride. As a byproduct, nitrosyl chloride bubbles out of the solution as a gas. The salt can be made more easily by reacting nitric acid with aluminum hydroxide.
Aluminium nitrate can also be made by metathesising aluminum sulfate and a nitrate salt with a suitable cation such as barium, strontium, calcium, silver, or lead. For example,
Al2(SO4)3 + 3 Ba(NO3)2 → 2 Al(NO3)3 + 3 BaSO4
Uses
Aluminium nitrate is commonly used in the production of other aluminium compounds, such as aluminium hydroxide and aluminium oxide, which are used in a wide range of industrial applications. It is also used in the manufacturing of catalysts and as a mordant in textile dyeing.
Aluminium nitrate is a powerful oxidizer. It is used in leather tanning, antiperspirants, corrosion inhibitors, uranium extraction, petroleum refining, and as a nitrating agent. There are numerous applications for nonahydrate and other hydrated aluminum nitrates. These salts are used to make alumina, which is then used to make insulating papers, cathode ray tube heating elements, and transformer core laminates. Actinide elements are extracted using hydrated salts as well.
Safety issue
In addition to its industrial applications, aluminium nitrate has some limited uses in laboratory settings, particularly in the synthesis of other compounds. However, it can be corrosive and toxic if ingested or inhaled, so appropriate safety measures should be taken when handling it.