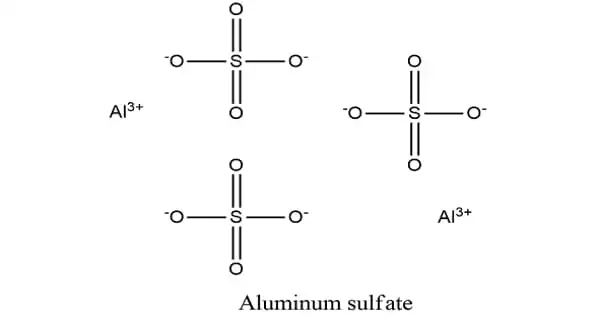
Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is primarily employed as a coagulating agent (increasing particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, as well as in paper manufacture.
The anhydrous form occurs naturally as the rare mineral millosevichite, which can be found in volcanic regions and on burning coal-mining waste dumps. Aluminium sulfate is rarely, if ever, seen as the anhydrous salt. It can produce a variety of hydrates, the most prevalent of which being hexadecahydrate Al2(SO4)3•16H2O and octadecahydrate Al2(SO4)3•18H2O. The heptadecahydrate, whose formula is [Al(H2O)6]2(SO4)3•5H2O, occurs naturally as the mineral alunogen.
Aluminium sulfate is sometimes called alum or papermaker’s alum in certain industries. However, the name “alum” is more commonly and properly used for any double sulfate salt with the generic formula XAl(SO4)2·12H2O, where X is a monovalent cation such as potassium or ammonium.
Properties
Aluminium sulfate is a white, crystalline, hygroscopic solid. The anhydrous salt has a density of 2.672 g/mL, while the octadecahydrate has a density of 1.62 g/mL. Its melting points are 770 oC (anhydrous salt) and 86.5 oC (aqueous salt) (octadecahydrate salt). It is highly soluble in hot water but less so in cold.
Aluminium Sulfate is water soluble but ethanol insoluble. Aluminium Sulfate has an odorless flavor that is somewhat astringent and pleasant. Aluminium Sulfate emits harmful sulphur oxide gases when it decomposes. Its solution is corrosive to aluminum. Aluminium sulfate is synthesized in the laboratory by combining aluminum hydroxide and sulfuric acid.
- Chemical formula: Al2(SO4)3
- Molar mass: 342.15 g/mol (anhydrous) and 666.44 g/mol (octadecahydrate)
- Appearance: white crystalline solid hygroscopic
- Density: 2.672 g/cm3 (anhydrous) and 1.62 g/cm3 (octadecahydrate)
- Melting point: 770 °C (anhydrous) and 86.5 °C (octadecahydrate)
- Solubility in water: 31.2 g/100 mL (0 °C); 36.4 g/100 mL (20 °C); 89.0 g/100 mL (100 °C)
- Solubility: slightly soluble in alcohol, dilute mineral acids

Chemical reactions
The compound decomposes to γ-alumina and sulfur trioxide when heated between 580 and 900 °C. It combines with water forming hydrated salts of various compositions.
Aluminium sulfate reacts with sodium bicarbonate to which foam stabilizer has been added, producing carbon dioxide for fire-extinguishing foams:
Al2(SO4)3 + 6 NaHCO3 → 3 Na2SO4 + 2 Al(OH)3 + 6 CO2
The foam stabilizer traps the carbon dioxide and produces a thick foam that floats on top of the hydrocarbon fuels, blocking access to atmospheric oxygen and suffocating the fire. Chemical foam could not be used on polar solvents like alcohol because the fuel would mix with and degrade the foam blanket. The carbon dioxide produced also served to propel the foam out of the container, whether it was a portable or stationary installation using hoselines. Chemical foam is regarded obsolete in the United States, having been replaced by synthetic mechanical foams such as AFFF, which have a longer shelf life, are more effective, and are more adaptable, yet it is still used in some countries such as Japan and India.
Applications
Aluminum sulfate is employed in a variety of everyday applications as well as in numerous critical sectors. Some of the most popular applications of aluminum sulfate can be found in the house. The chemical is commonly found in baking soda, while there is some debate about whether it is healthy to consume aluminum. Water treatment and purification is one of the most important applications for aluminum sulfate.
Uses of Aluminium Sulfate –
- In baking soda.
- In the dyeing of cloth.
- In making paper.
- In concrete as an accelerator and waterproofing agent.
- As the fireproofing agent.
- In firefighting foam.