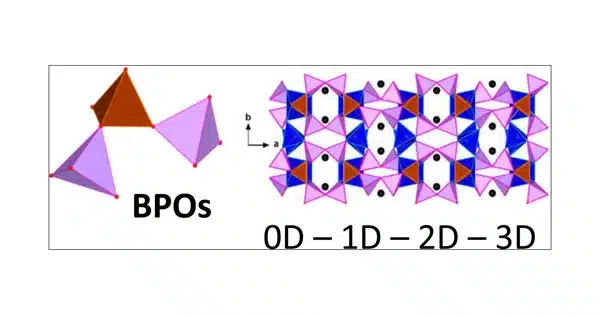
Borophosphates are an inorganic compound class made up of boron, phosphorus, and oxygen. Depending on the specific combination of boron and phosphorus atoms and how they are arranged in the crystal lattice, these compounds can have a wide range of structures and properties.
Borophosphates have been investigated for potential uses in catalysis, gas storage, and optoelectronics. Because of their high ionic conductivity and excellent electrochemical properties, they have also been investigated as potential materials for solid-state batteries and supercapacitors.
Borophosphates are mixed anion compounds that contain borate and phosphate anions that may be linked together by a common oxygen atom. Compounds with water or hydroxy groups can also be classified as compounds.
Properties
- Hardness: These are generally hard materials with high resistance to scratching and abrasion.
- Thermal stability: These have good thermal stability, which means they can withstand high temperatures without breaking down or decomposing.
- Electrical conductivity: These can be either insulators or conductors of electricity, depending on their chemical composition and crystal structure.
- Chemical resistance: These are generally resistant to chemical attack, making them useful in a variety of applications where exposure to corrosive substances is a concern.
- Magnetic properties: Some borophosphates exhibit magnetic properties, which make them useful in applications such as data storage.
Borophosphates are classified according to whether they are hydrated and their anion structure, which can be single, double, triple, isolated ring, isolated branched ring, a simple chain, branched chain, loop chain, layers, or three-dimensional network. The borate phosphates are single anion compounds with distinct borate and phosphate groups. Some borophosphate structures resemble silicates.
Related compounds include aluminophosphates, which have aluminum instead of boron, gallophosphates, with gallium in place of boron, and by substituting the phosphate: boroarsenates, boroantimonates, vanadoborate.
In addition, borophosphates have shown promise as potential hosts for radioactive waste disposal, as their crystal structure can trap and immobilize certain types of radioactive isotopes. Overall, it represents a versatile class of materials with a wide range of potential applications in various fields of science and technology.