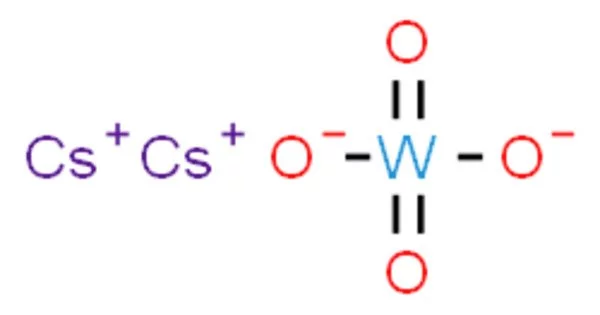
Caesium tungstate, also known as cesium tungstate, is an inorganic chemical compound that forms a very dense liquid in solution. Because diamond sinks in it, the solution is used in diamond processing, whereas most other rocks float. A tungstate is a tungsten oxyanion-containing compound.
Rare-earth tungstates are negatively charged oxytungstate ions that have been extensively studied in the fields of luminescence and catalysis. Tungstate ions can also easily polymerize to form polytungstates. However, only a few materials containing essential tungstates have been reported, limiting the range of applications of tungstate-based materials in modern industries.
Properties
Caesium tungstate forms colorless crystals, which are strongly hygroscopic. A phase transition from orthorhombic to hexagonal crystal system occurs at 536°C.
- Chemical formula: Cs2WO4
- Molar mass: 513.65 g/mol
- Melting point: >350 °C
- Appearance: White to off-white powder
- Solubility in H2O: Soluble

Preparation
Caesium tungstate can be made by reacting caesium chloride (CsCl) with silver tungstate (Ag2WO4) or by reacting tungstic acid with caesium hydroxide.
When single and binary tungstates are combined with cocatalysts or another visible light catalyst, the degradation efficiency of these ternary hybrids is increased. When compared to a binary heterojunction, these ternary tungstate heterojunctions can more efficiently separate and transfer photogenerated charge carriers, increasing the range of light absorption.
Uses
Cesium tungsten oxide is used to create the best infrared transparent barrier transparent insulation coating. The recombination of photo-induced electron-hole pairs, as well as the single or binary metal tungstates’ narrow light response range to the solar spectrum, limits their wide application.