Extracting CO2 from the atmosphere is one of the most effective ways to reverse climate change without relying on expensive technologies, complicated tax schemes, or denying billions of people access to the energy they require to live a decent life. If you could then turn it into gasoline, diesel, or jet fuel, you’d be killing two birds with one stone. Carbon Engineering is the name of that stone. We are left with using our enormous brains, which got us into this mess in the first place, to try to wiggle our way out of it because we are failing to reduce global carbon emissions at all.
A-CuTi@Cu, a new electrocatalyst, converts carbon dioxide (CO2) into liquid fuels. According to a team of Chinese researchers who published their findings in the journal Angewandte Chemie, active copper centered on an amorphous copper/titanium alloy efficiently produces ethanol, acetone, and n-butanol.
The majority of our global energy needs are still met by burning fossil fuels, which contributes to the greenhouse effect through CO2 emissions. To combat global warming, we must look for ways to use CO2 as a raw material for basic chemicals. A climate-neutral, artificial carbon cycle could be established through electro catalytic CO2 conversion using renewable energy. Excess energy generated by photovoltaics and wind energy could be stored by electro catalytically producing fuels from CO2. These could then be disposed of as needed. Conversion to liquid fuels would be advantageous due to their high energy density and ease of storage and transportation. However, the electro catalytic formation of products with two or more carbon atoms (C2+) is very challenging.
A-CuTi@Cu, a new electro catalyst, converts carbon dioxide (CO2) into liquid fuels. Active copper centered on an amorphous copper/titanium alloy efficiently produces ethanol, acetone, and n-butanol.
Fei Hu, Tingting Kong, Jun Jiang, and Yujie Xiong from Foshan University
A team led by Fei Hu, Tingting Kong, Jun Jiang, and Yujie Xiong from Foshan University (Foshan, Guangdong), the University of Science and Technology of China (Hefei, Anhui), and Xi’an Shiyou University (Xi’an, Shaanxi has developed a novel electro catalyst that efficiently converts CO2 to liquid fuels with multiple carbon atoms (C2-4). Ethanol, acetone, and n-butanol are the main byproducts.
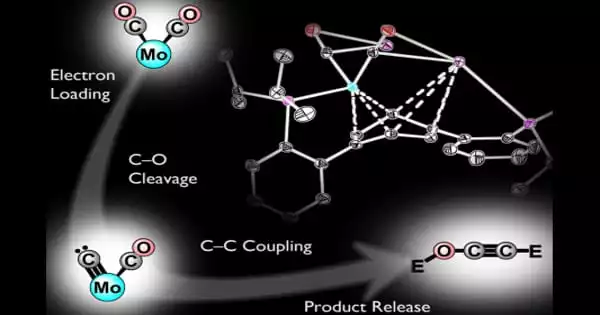
Thin ribbons of a copper/titanium alloy are etched with hydrofluoric acid to remove the titanium from the surface to create the electro catalyst. This yields a material known as a-CuTi@Cu, which has a porous copper surface on an amorphous CuTi alloy. It has catalytically active copper centers with exceptional activity, selectivity, and stability for reducing CO2 to C2+ products (total faradaic efficiency of about 49 percent at 0.8 V vs. reversible hydrogen electrode for C2-4, and it is stable for at least three months). Pure copper foil, on the other hand, produces C1 products but almost no C2+ products.
A multistep electron-transfer process via various intermediates is involved in the reaction. The inactive titanium atoms beneath the surface play an important role in the new electro catalyst, increasing the electron density of the Cu atoms on the surface. This stabilizes CO adsorption, a key intermediate in the formation of multi carbon products, allows for high CO coverage of the surface, and lowers the energy barrier for di- and trimerization of CO as new carbon-carbon bonds form.
NASA has developed a new technology that uses solar-powered, thin-film devices to convert the greenhouse gas carbon dioxide (CO2) into fuel. Metal oxide thin films are created in order to create a solar-powered photo electrochemical cell. By converting CO2 to fuel before it is emitted into the atmosphere, this technology can mitigate the effects of the world’s major fuel source for the foreseeable future, fossil fuels. This new nanomaterial thin-film device offers a low-cost, simple fabrication route for commercializing the technology in the sustainable energy market. More importantly, by recycling CO2 into fuels that are compatible with all existing fuel utilities, it results in a zero carbon footprint. This is accomplished by using solar power to convert CO2 into a usable fuel in a small device.
A photo electrochemical cell made of thin metal oxide films is used in this technology. The reaction is carried out using sunlight (primarily the ultraviolet (UV), visible, and infrared (IR) portions) and low-cost titanium dioxide composites. The device can be used to capture carbon dioxide from industrial processes before it is released into the atmosphere and convert it to a useful fuel like methane. These devices have low manufacturing and material costs, allowing them to be deployed in the commercial market. They can be made to be extremely compact and efficient, and they can be used in sensor and detector applications.