Consequences of the Absorption of Light
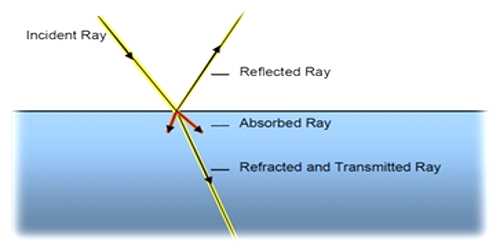
Light is a form of energy. According to the principle of photochemical activation, only that part of the light which is absorbed by any system can cause photochemical change or reaction. However, this is not essential that every time, the energy which is absorbed by any system can bring about a photochemical reaction. Absorption of light means absorption of radiation energy. Absorption of radiation energy by a substance may lead to the following changes:
(i) Thermal change: The kinetic energy of the molecules may be increased giving rise to an increase in temperature. i.e., heat will be generated.
(ii) Excitation: The internal energy of the molecules or atoms may be raised. This will result in elevating the electrons to higher energy levels. In spectroscopic terminology, it is assumed that the atoms or molecules are raised from the ground state energy levels to the excited state energy levels.
(iii) Dissociation: The molecule breaks down to form smaller molecules, atoms or free radicals. Excitation and dissociation may occur simultaneously.
(iv) Emission: Part of the absorbed radiation may be re-emitted giving rise to fluorescence or phosphorescence.
The first of the four processes are not important when visible and ultraviolet light are considered. When the light of shorter wavelengths is used, in addition to the above processes ionization and physical interaction of the photons and matter become also prominent.