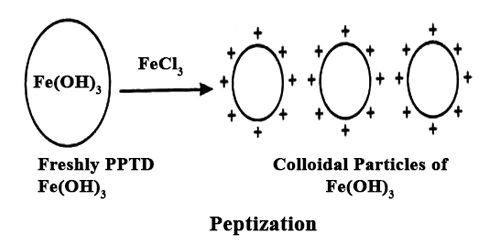
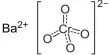
Peptization is the process by which a coagulum or a precipitate is dispersed to a colloidal state. It is the reverse process of coagulation. It is the process responsible for the formation of a stable dispersion of colloidal particles in the dispersion medium.
If a freshly prepared precipitate of silver bromide is washed in a controlled method, the precipitate can be simply dispersed in water to give a sol. Several sulphides, particularly of arsenic and cadmium, are simply peptized by a further treatment of H2S. Small but suitable amount of electrolytes, weak or strong, is necessary for peptization and following constancy of the sol.
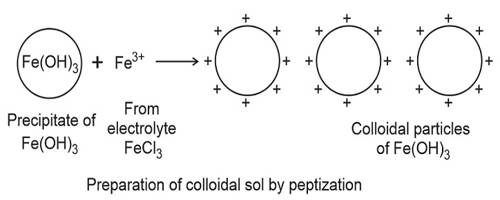
The electrolyte used in this procedure is called as a peptizing agent. It is also used in nanoparticle synthesis to make a large grouping of particles split into many major particles. This is done by changing the surface properties, applying a charge, or by adding a surfactant.
Peptization process is possible in case of the complex situation. The word Peptization comes from peptic digestion in which large solid particles of food are disintegrated to small ones prior to absorption and metabolism.