Laboratory Preparation of Chloroform from acetone:
CHCl3 can be prepared in the laboratory by reaction of bleaching powder with ethanol or acetone. In this reaction, bleaching powder acts as an oxidizing chlorinating and hydrolyzing agent. A mixture of acetone, bleaching powder, and water, when heated produce chloroform. The reaction occurs in 3 steps.
- Step-1: At first bleaching powder and water react to form chlorine and lime water [Ca(OH)2]
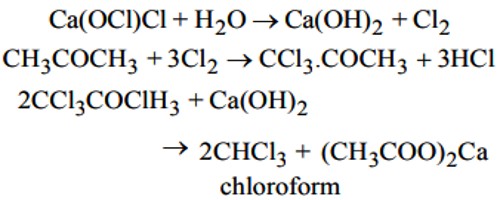
Ca(OCl)Cl (bleaching powder) + H2O → Ca(OH)2 Cl2
- Step-2: Then the chlorine reacts with the acetone and by its chlorination 1, 1, 1-tri-chloroacetone is formed.
CH3-CO-CH3 (acetone) + Cl2 → Cl3C-CO-CH3 (1, 1, 1-trichloro acetone) + HCl
- Step-3: The produced trichloro acetone reacts with lime forming chloroform.
Cl3C-CO-CH3 + Ca(OH)2 → CHC13 (chloroform) + (CH3COO)2Ca
Procedure: The preparation is ranted out in the following manner.
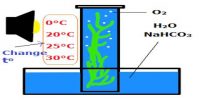
A paste of bleaching powder made with a little water is taken in a round-bottomed flask fitted with a Liebig condenser by means of a delivery tube. There is a receiver at the other end of the condenser. Alcohol is added slowly into the flask. The mouth of the flask is then closed and the flask is heated on a sand bath. The cold water circulating through the jacket of the condenser tube cools the heavy vapor of the chloroform which is collected as a liquid in the receiver, some water also condensed with chloroform.
Another way – The flask is then fitted with a water condenser at the other end of which a receiver is joined. The flask is then placed over a sand bath and heated till a mixture of chloroform and water distills over. The mixture from the receiver is transferred to a separating funnel and the lower layer of chloroform is separated.
Purification:
Hence, labs prepared Chloroform is impure and may contain acids and water as impurities. The chloroform thus obtained contains unreached alcohol, hydrochloric acid, etc. as impurities. The distillate from the receiver is taken in a separating funnel. Chloroform being heavier than water forms the lower layer which is separated by a separating funnel. It is then washed with dilute NaOH solution to remove alcohol and HCl and then with water. The chloroform thus freed from HCl and alcohol is dried over fused CaCl2 redistilled (bp. 620C) when pure chloroform is obtained as distillate.
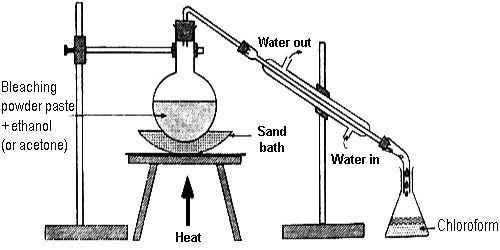
Fig: Laboratory Preparation of Chloroform
Another Way – Chloroform obtained by heating bleaching powder paste with acetone or ethanol is not in pure form. To remove impurities, it is passed through dilute caustic soda solution and washed with water in a separating funnel. The chloroform is then dried over anhydrous CaCl2 and redistilled between the temperature 60°C-65°C.
Uses of Chloroform
Chloroform is a sugary smelling liquid which is used for the following a purpose.
- It is used as the solvent for the manufacture of dyes and pesticides.
- Chloroform generally contains heavy metal like deuterium which is used for solvent as Nuclear Magnetic Resonance (NMR) Spectroscopy.