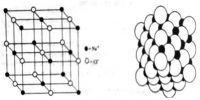
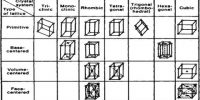
Semiconductors in Solid Materials
The term semiconductor refers to those which have the weak conductivity at low temperatures but show increased conductivity as the temperature is raised. In such solids, the topmost filled valence band is separated from empty conduction hand by a small energy gap. Since the energy gap is smaller an appreciable number of electrons can be promoted from the top of the valence band into the bottom of the conduction band and the material behaves like a conductor.
![]()
Figure: energy bands in a semiconductor (forbidden hand is small)
Based on their origin the semiconductors are broadly divided into two classes:
(i) Intrinsic semiconductor and
(ii) Extrinsic semiconductor.
A semiconductor in which the concentration of charge carriers, electrons or holes, is characteristic of the material itself rather than of the presence of any impurities or structural defects of the crystal is called an intrinsic semiconductor. The carrier concentration, and hence the conductivity of intrinsic semiconductor, is very sensitive to temperature and depends strongly on the energy gap. The energy gap ranges from a fraction of 1.0 eV to several eV. Silicon and germanium are typical intrinsic semiconductors. At 3000 K the thermal energy of the valence band electrons is not sufficient to allow many of them to cross the forbidden energy gap and go to the conduction band. These have poor conducting power. When the temperature is raised, many electrons will move up to the conduction band and one rise in the conductivity. Such excitation of the electrons to the conduction band may also be achieved by allowing radiation of short wavelength (example: high frequency or energy) to be incident on the solid.
Sometimes the conductivity of a pure semiconductor may be significantly enhanced by incorporating a trace amount of impurity into the crystal structure to produce what is known as extrinsic semiconductivity. This type of semiconductor is also known as impurity semiconductors. The impure atoms exist at lattice sites in the crystal in place of the atoms in the crystal. The effect of the impure atoms is to introduce new energy levels. These levels may lie in a gap between a filled band and an empty band. Electrons may be excited to the conduction band and these will contribute significantly to the conduction. For example, incorporation of a trace amount (parts per billion levels) of gallium or arsenic into pure silicon and/or germanium increases conductivity by 2 to 3 order of magnitude.