Ideal Gas
The gases which follow fundamental postulates of kinetic theory of gases and at all temperatures and pressures simultaneously obey both Boyle’s law and Charles’s law are called ideal gases.
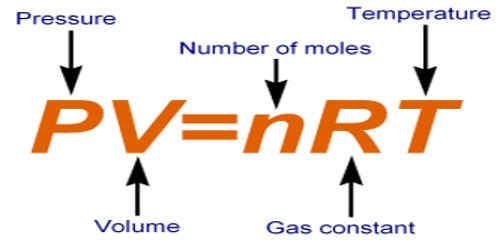
An ideal gas is a gas whose pressure P, volume V, and temperature T are related by the ideal gas law: PV = nRT,
where n is the number of moles of the gas and R is the ideal gas constant.
In reality there is no existence of a gas which follows all these fundamental postulates correctly. So, it is seen that real gases differ from ideal gases. Only at a low pressure and at a very high temperature a gas follows this relation. In reality, no gasses are known to us that follow correctly the ideal gas equation. All gases do not behave as ideal gases. Ideal gas is a hypothetical concept only.
The term ideal gas refers to a hypothetical gas composed of molecules which follow a few rules:
- Ideal gas molecules do not attract or repel each other. The only interaction between these gas molecules would be an elastic collision upon impact with each other or an elastic collision with the walls of the container.
- Ideal gas molecules themselves take up no volume. The gas takes up volume since the molecules expand into a large region of space, but the Ideal gas molecules are approximated as point particles that have no volume in and of themselves.