Intermolecular Force in State of Matter
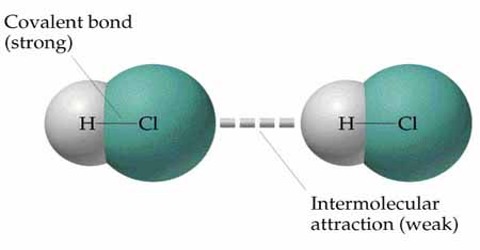
Before discussing elasticity we need to know the structure of matter and why elasticity is produced in matter. Normally molecules having covalent bond have weak attractive force between molecules and thermal vibration can easily overcome this attractive force. Weak attractive force between covalent molecules is called intermolecular force or energy. In other words, atoms in covalent molecules attract other atoms with a weak force which is called the interatomic attractive force. While forming matter, molecules remain close to each other and there is a small vacant space between atoms. This distance is about 10-9 m to 10-19 m. The molecules in this distance attract each other with a weak force. This is the intermolecular force. This intermolecular force that keeps molecules of a solid material bounded together is of electrical in nature. Due to the interaction of this electrical force is generated due to the interaction of the charged ions by which the molecules are created. We know that intermolecular force at between molecules of all materials. Force active between molecules of a metal is called cohesive force. It is true that in normal condition molecules of the Crystal have lowest potential energy. This state is their stable state. In this situation, net intermolecular force active between molecules is zero.
If a body is expanded by applying force, the intermolecular space increases and according to the third law of motion or due to inertia molecules tries to regain their original position. Similarly, if a body is contracted by applying external force, intermolecular space decreases and the body becomes squeezed. Again, due to inertia or according to Newton’s third law of motion the body tries to go back to its original shape or position. Due to this, elastic property is created in bodies.
Depending on the intermolecular space materials are classified into two groups, viz. (1) solid and (2) fluid.
Fluids are classified again into two groups, viz— non-compressible fluid, e.g. liquid and compressible fluid, e.g., gas. Besides, due to extremely high temperature gaseous materials are ionized. Here equal numbers of positive and negative ions are produced. This state of matter is called plasma state.