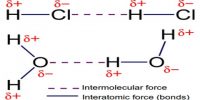
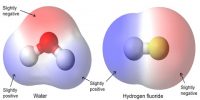
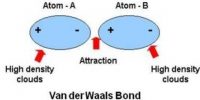
Ionic Bond
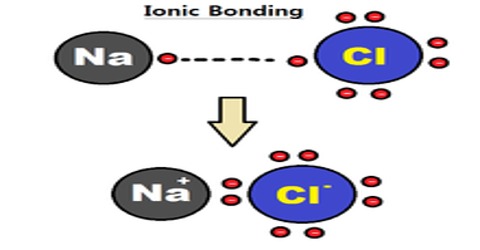
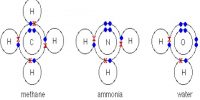
While mixing a metal with a non-metal normally one or more electrons are donated by metal atom to non-metal atom. Consequently metal atom becomes positively charged ions and non-mental atom becomes negatively charged ions. These two ions being oppositely charged electrical attraction between them is created. This attraction keeps both ions bounded together. On the other hand, ionic crystal is formed by the combination of these positive and negative ions. Distribution of ions is such that attractive force between the oppositely charged ions becomes stronger. Ionic bond is the result of mutual interaction of oppositely charged ions. An ion tries to assemble maximum possible number of oppositely charged ions around it. As those ions are of similar charge, there exists repulsive force and finally it reaches to a compromise to form a crystal. This is how ionic bond is formed in crystal.
Due to the chemical interaction of metallic and non-metallic elements electrons from the outer shell of a metal is transferred to the outer shell of non-metal and the bond formed by the electrical attraction of positive and negative ions, is called ionic bond.
The elements created by ionic bond is called ionic element.
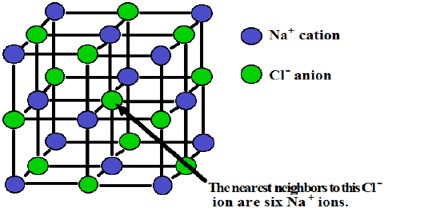
Example: In figure ionic bond of sodium chloride (NaCl) has been shown. For the formation of smut bond following three conditions are needed:
(i) First element should have lower ionization energy,
(ii) Second element should have higher electron affinity,
(iii) Higher lattice energy of the compound formed.