Peroxides are a class of chemical compounds that contain an oxygen-oxygen single bond (O-O). Peroxides are a class of compounds with the formula ROOR, where R can be any element. The most common peroxide is hydrogen peroxide (H2O2), also known as “peroxide” in colloquial parlance. The OO group in a peroxide is known as the peroxide group or peroxy group (also known as the peroxo group or peroxyl group).
The term was coined by Thomas Thomson in 1804 for an oxide with the greatest amount of oxygen. This bond is frequently more reactive than other oxygen-containing functional groups. The general formula for peroxides is R-O-O-R’, where R and R’ are organic groups. Peroxides can be organic or inorganic in nature. It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well.
In addition to hydrogen peroxide, some other major classes of peroxides are:
- Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate.
- Main group peroxides, compounds with the linkage E−O−O−E (E = main group element).
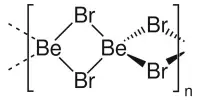
- Metal peroxides, examples being barium peroxide (BaO2), sodium peroxide (Na2O2) and zinc peroxide (ZnO2).
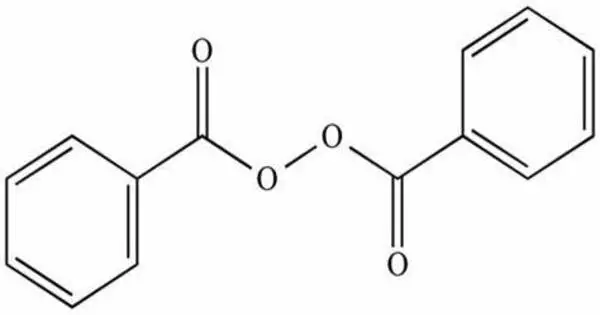
- Organic peroxides, compounds with the linkage C−O−O−C or C−O−O−H. One example is tert-butylhydroperoxide.
Here are a few key points about peroxides:
- Hydrogen Peroxide (H2O2): This is one of the most well-known peroxides. It’s a pale blue liquid that appears colorless in a dilute solution. Hydrogen peroxide is a powerful oxidizer and can act as both a reducing agent and an oxidizing agent. It’s commonly used as a disinfectant and bleaching agent.
- Organic Peroxides: These are compounds where the peroxide group is part of an organic molecule. Organic peroxides are widely used in industries, especially in the production of polymers, resins, and pharmaceuticals. They can be highly reactive and, in some cases, prone to decomposition, which can be hazardous.
- Safety Considerations: Peroxides should be handled with caution due to their reactivity. In certain conditions, some peroxides can be explosive or initiate polymerization reactions. Stabilizers are frequently used in the storage and transportation of peroxides to reduce the risk of decomposition.
- Radical Reactions: Peroxides participate in radical reactions, which occur when a molecule gains or loses an electron. These reactions are crucial in a variety of chemical processes, including polymerization reactions.
- Polymerization: Peroxides are commonly used as polymerization initiators for certain monomers. They, for example, play an important role in the production of materials such as polyethylene and polystyrene.