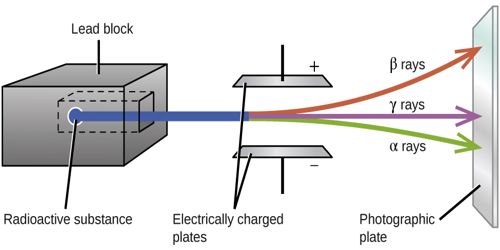
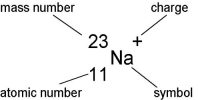
We know, from radioactive substances three types of rays are emitted. These are α, β, and y-rays. Here Alpha-rays (α-rays) properties are described below.
Their different properties are described below.
Properties of α-rays:
(1) These rays consist of some moving charged particles. Mass of each particle is 6.6 x 10-27 kg. The mass of an α-particle is about four times greater than that of a hydrogen atom.
(2) α-rays carry positive charges. An α-particle has a charge of 3.2 x 10-19 C.
(3) Each α-particle is nothing but a doubly ionized helium atom.
(4) These are deflected by both the electric and the magnetic fields. This proves that α-particles are charged particles. From the direction of deflection, it is proved that α-particles are positively charged.
(5) α-particle can ionize gases. Their ionization power is more. The ionizing power of α-rays is about 100 times greater than that of β-rays and 10.000 times greater than that of γ-rays.
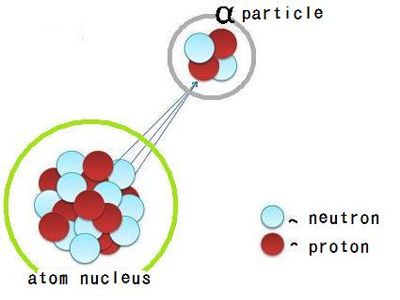
Fig: radioactive α-Rays
(6) α-particles affect photographic plates.
(7) These rays are easily absorbed by substances. That means, their penetrating power is very small. Compared to β-rays and γ-rays their penetrating power is much less.
(8) α-rays produce fluorescence in zinc sulfide or barium platinocyanide.
(9) Their range in air is from 0.027 m to about 00.09 m.
(10) The velocity of emission of α-particles varies from source to source within a range of 1.4 x 107 ms-1 to 1.9 x 107 ms-1. This velocity of emission depends upon the nature of the radioactive element,
(11) α-rays produce ulcers when they are incident on the human body. It is very difficult to cure such ulcers.
(12) α-particles are scattered in all directions when they pass through a very thin sheet of metal or mica.