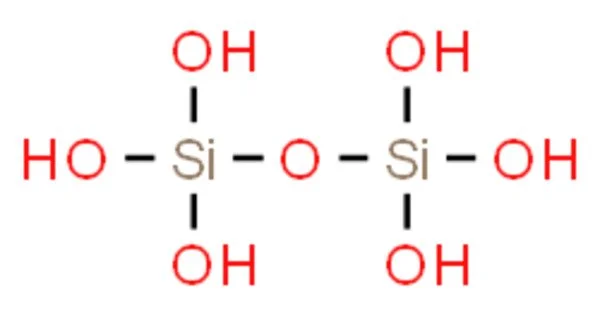
Pyrosilicic acid is a chemical compound with the formula H6Si2O7 or (HO)3SiOSi(OH)3. It is also known as dipyrosilicic acid. It is one of the silicic acids and has pyrosilicate as its conjugate base. It is a polymeric oxide of silicon, meaning it contains multiple silicon atoms bonded together in a complex structure. The compound is not well-characterized in its pure form because it tends to polymerize and form various silicate species in aqueous solutions.
Pyrosilicic acid can be formed by the dehydration of orthosilicic acid (H4SiO4), which is commonly found in water and soil. The process involves the elimination of water molecules, leading to the formation of polymeric silicate structures. The exact nature of these structures can vary depending on factors such as pH, temperature, and the presence of other ions.
Properties
- Chemical formula: H6O7Si2
- Molar mass: 174.211 g·mol−1
- Conjugate base: Pyrosilicate
- Appearance: It is not a well-defined solid; it is usually encountered in solution form.
- Solubility: It is soluble in water, and its aqueous solution is acidic.
- Acidity: It is a weak acid, and its acidity arises from the dissociation of protons (H+) from the water molecules.
Structure: The molecular structure of pyrosilicic acid involves two silicon atoms and several oxygen and hydrogen atoms.
Chemical Stability: In general, pyrosilicic acid is not very stable and tends to undergo further reactions, including polymerization or hydrolysis.
It was synthesized, using nonaqueous solutions, in 2017.
H6Si2O7 (aq) ⟶ 2SiO2 (s) + 3H2O
Pyrosilicic acid may be present in seawater and other natural waters at very low concentrations. Compounds formally derived from it, such as sodium pyrosilicate, are found in the sorosilicate minerals.
In natural environments, silicic acids play a role in the geochemical cycling of silicon, which is an essential element for various organisms. Silicon is involved in the formation of silicate minerals and is important for the growth of diatoms and other siliceous organisms.