Simple molecules
Most covalently bonded substances exist as simple small or medium sized molecules, rather than as giant structures. When simple molecules are heated moderately, the atoms do not get enough kinetic energy to break the strong covalent bonds, and so most molecules do not break apart (decompose) on heating.
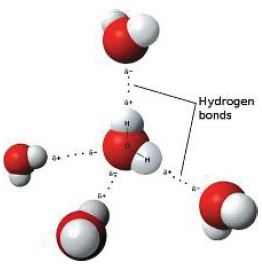
The forces between molecules – London dispersion or dipole-dipole are much weaker than the covalent bonds. Just a little heat gives enough kinetic energy to separate each molecule from neighbouring molecules. When a molecular substance melts, only the weak intermolecular bonds are broken.
The size of the molecule affects the melting and boiling points, as larger, heavier molecules are more difficult to separate. Small molecules (H2, H2O) have low melting and boiling points, are gases or liquids at room temperature. Larger molecules (C9 H8O4) aspirin, C8H9N4O2 caffeine) have higher melting points (but still << ionic) and are solids at room temperature.
Stronger intermolecular forces (such as dipole- dipole or hydrogen bonding) are more difficult to break. Polar molecules such as water have higher melting and boiling points than non-polar molecules such as methane of similar size.