The lead storage cell (a rechargeable cell) consists of electrodes of lead alloy grids; one electrode is packed with a spongy lead to form the anode, and the other electrode is packed with lead dioxide to form the cathode.
Storage cell can be recharged by passing electric current and used again and again. Examples of storages or secondary cells are, lead storage battery and nickel – cadmium storage cell.
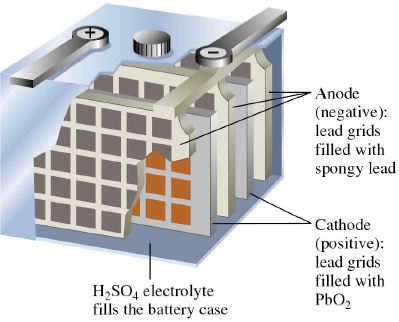
There are two classes of secondary cells or storage :
(i) nonrechargeable or “primary” cells, for example typical cheap throwaway flashlight batteries, and
(ii) rechargeable or “secondary” cells, for example an automotive lead-acid battery.









