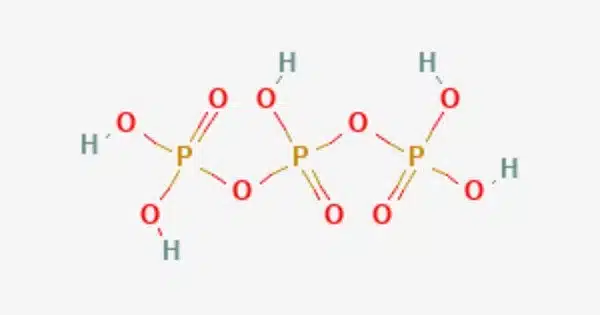
Triphosphoric acid (sometimes tripolyphosphoric acid) is a condensed form of phosphoric acid having the formula H5P3O10. It is frequently discussed in the context of phosphoric acids, which encompass a variety of phosphorus-containing acids with varying numbers of phosphate groups (HPO3, H2PO4, H3PO4, and so on). H4P2O7, also known as diphosphoric acid, is the next polyphosphoric acid following pyrophosphoric acid in the phosphoric acid family.
Triphosphoric acid is a polyphosphoric acid, which indicates that its structure contains multiple phosphate groups. Esters of triphosphoric acid include compounds such as ATP (adenosine triphosphate). When compared to phosphoric acid, it is a less frequent and less stable chemical.
Properties
- Molecular Weight: 182.00 g/mol
- Appearance: It is typically found as a colorless, viscous liquid.
- Acidity: It is highly acidic and can donate up to four protons (H+) in aqueous solution.
- Hygroscopic: It readily absorbs moisture from the air, making it a hygroscopic compound.
- Reactivity: It is a strong dehydrating agent, and it can react with water to form polyphosphoric acids.
- Stability: It is less stable than orthophosphoric acid (H3PO4) and can easily decompose under certain conditions.
Triphosphoric acid is not available in crystalline form. Triphosphoric acid accounts for approximately 20% of the equilibrium mixture with an overall composition similar to H5P3O10. A pure species solution can be created by ion exchange of the sodium salt, sodium triphosphate, at 0 °C.
Uses
Triphosphoric acid is rarely utilized directly in industrial or laboratory settings. Instead, it is frequently employed as an intermediary in the synthesis of different phosphate compounds and polyphosphoric acids, which are then used to manufacture detergents, food additives, and other chemicals.
Safety
Because triphosphoric acid is corrosive, it should be handled with caution. On touch, it might cause skin and eye discomfort.