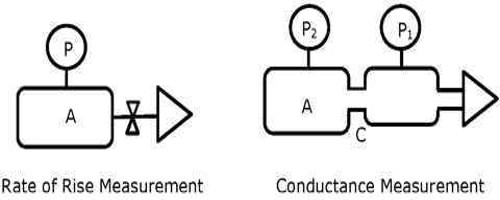
Applications of Conductance Measurements
The conductance of electrolytes depends on the number of ions and their speeds. The measurements of conductance of electrolytic solutions have been utilized for determining –
(a) the endpoints of acid-base titrations,
(b) the endpoints of precipitation titrations;
(c) the solubility of sparingly soluble salts and
(d) the kinetics of reactions.
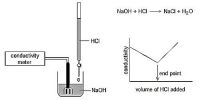
For conductometric titration experiments, a known volume of the solution to be titrated is placed in a beaker and a conductivity cell dipped into it. The conductivity cell is now connected to one end of the Wheatstone’s bridge. The other solution is then added to the solution in the beaker in installments and the conductance measured after each addition. This is continued beyond a sharp change in conductance value. The conductance values are then plotted against the volumes of titrated added. In each case, the straight-line portions of the graph are extrapolated and the point at which the straight lines intersect is taken as the end pony of the titration.
In natural waters, conductivity is mostly used to approximation the concentrations of dissolved salts in the water, which can provide insights into processes affecting the water. In coastal areas, the conductivity of water might change with assimilation with saltwater, and the conductivity of water might rise when it becomes contaminated with road salt in areas with cool climates.
Common Application of conductance measurements:
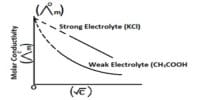
- Determination of dissociation constant of weak electrolytes from conductance measurements.
- Weak electrolytes like HAc dissociation according to – Determination the solubility of a sparingly soluble salt by conductance measurement.
Water Treatment and Industrial Applications
Water treatment may be used to make water safe to drink or proper for manufacturing use. In many industrial applications, scale or corrosion might be an apprehension. Conductivity might also be used to monitor the usefulness of desalinization, which is another water conduct procedure that removes salts to make water drinkable or useable for manufacturing processes.
In other industrial applications, conductivity measurements might be used to identify leaks, where the leaking water might have a superior conductivity. Conductivity might also be used to check the efficiency of rinsing procedures, where a low conductivity of water in contact with the rinsed object indicates an efficient rinse.
Agricultural and Hydroponics Applications
For irrigation, the salinity of the water is a significant factor. If the salinity is too high, salts will build up in the soil as the water evaporates which might demean soil eminence and inhibit plant growth.
Conductivity can also be used to observe nutrient concentrations in liquid fertilizers.
Comparable to fertilizer application, conductivity is used in hydroponics to monitor the concentrations of nutrient solutions. If the conductivity gets too high, indicating a nutrient concentration at toxic levels, plants might be debilitated or die. Low conductivities can designate insufficient nutrient supply. Conductivity monitoring can be used as a component of mechanized nutrient supply systems.
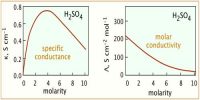
Conductance offers a very simple and convenient mean for determining the solubility of a sparingly soluble salt such as AgCl, etc. dissolved to a very small extent. That is why these salts are called sparingly soluble salts.