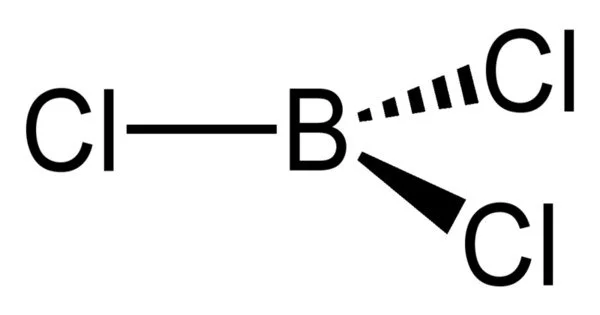
Boron trichloride is an inorganic compound with the formula BCl3. It appears as a colorless gas with a strong odor. This colorless gas is used as a reagent in organic synthesis. It is highly reactive to water.
Boron trichloride is a Lewis acid that forms stable addition compounds with donors such as ammonia and amines and is used in the laboratory to promote reactions that liberate these donors. The compound is useful in industry as a source of pure boron (reduction with hydrogen) for the electronics industry. It is also used to make boranes by reacting with metal hydrides.
Properties
Boron trichloride is an odorous colorless gas. It reacts violently with water, releasing hydrochloric and boric acid upon decomposition and hydrolysis. It has a strong, unpleasant odor. Boron and boron compounds can be found in industries that make special glass, washing powder, soap and cosmetics, leather, cement, and other products.
- Chemical formula: BCl3
- Molar mass: 117.17 g/mol
- Appearance: Colorless gas, fumes in air
- Density: 1.326 g/cm3
- Melting point: −107.3 °C (−161.1 °F; 165.8 K)
- Boiling point: 12.6 °C (54.7 °F; 285.8 K)
- Solubility in water: hydrolysis
- Solubility: soluble in CCl4, ethanol
- Magnetic susceptibility (χ): -59.9·10−6 cm3/mol
- Molecular shape: Trigonal planar (D3h)
Production and structure
Boron reacts with halogens to give the corresponding trihalides. Boron trichloride is, however, produced industrially by direct chlorination of boron oxide and carbon at 501 °C.
B2O3 + 3 C + 3 Cl2 → 2 BCl3 + 3 CO
The carbothermic reaction is analogous to the Kroll process for the conversion of titanium dioxide to titanium tetrachloride. In the laboratory BF3 reacted with AlCl3 gives BCl3 via halogen exchange. BCl3 is a trigonal planar molecule like the other boron trihalides, and has a bond length of 175pm.
A degree of π-bonding has been proposed to explain the short B− Cl distance although there is some debate as to its extent. It does not dimerize, although NMR studies of mixtures of boron trihalides shows the presence of mixed halides. The absence of dimerisation contrasts with the tendencies of AlCl3 and GaCl3, which form dimers or polymers with 4 or 6 coordinate metal centres.
Reactions
BCl3 hydrolyzes readily to give hydrochloric acid and boric acid:
BCl3 + 3 H2O → B(OH)3 + 3 HCl
Alcohols behave analogously giving the borate esters, e.g. trimethyl borate.
Ammonia forms a Lewis adduct with boron trichloride.
As a strong Lewis acid, BCl3 forms adducts with tertiary amines, phosphines, ethers, thioethers, and halide ions. Adduct formation is often accompanied by an increase in B-Cl bond length. BCl3•S(CH3)2 (CAS# 5523-19-3) is often employed as a conveniently handled source of BCl3 because this solid (m.p. 88-90 °C) releases BCl3:
(CH3)2S·BCl3 ⇌ (CH3)2S + BCl3
The mixed aryl and alkyl boron chlorides are also of known. Phenylboron dichloride is commercially available. Such species can be prepared by the redistribution reaction of BCl3 with organotin reagents:
2 BCl3 + R4Sn → 2 RBCl2 + R2SnCl2
Reduction
Reduction of BCl3 to elemental boron is conducted commercially In the laboratory, when boron trichloride can be converted to diboron tetrachloride by heating with copper metal:
2 BCl3 + 2 Cu → B2Cl4 + 2 CuCl
B4Cl4 can also be prepared in this way. Colourless diboron tetrachloride (m.p. -93 °C) is a planar molecule in the solid, (similar to dinitrogen tetroxide, but in the gas phase the structure is staggered.
Uses
Boron trichloride is a precursor to the production of elemental boron. It is also used to remove nitrides, carbides, and oxides from molten metal in the refining of aluminum, magnesium, zinc, and copper alloys. It’s been used as a soldering flux for aluminum, iron, zinc, tungsten, and monel alloys.
BCl3 is a reagent used in the synthesis of organic compounds. It cleaves C-O bonds in ethers similarly to the corresponding bromide.
Safety
BCl3 is an aggressive reagent that can form hydrogen chloride upon exposure to moisture or alcohols. The dimethyl sulfide adduct (BCl3SMe2), which is a solid, is much safer to use, when possible, but H2O will destroy the BCl3 portion while leaving dimethyl sulfide in solution.