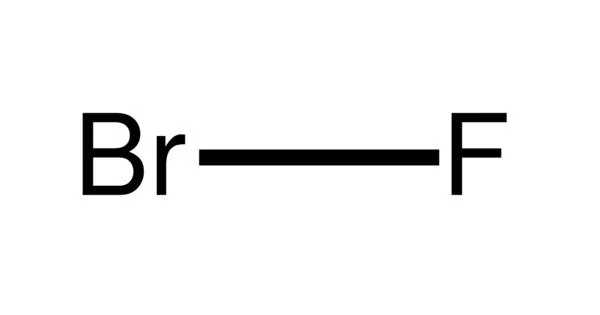Bromine monofluoride (BrF) is an extremely unstable interhalogen compound. It is created by reacting bromine trifluoride (or bromine pentafluoride) with bromine. There are two atoms in the Bromine monofluoride molecule (s). There are one or more fluorine atoms and one bromine atom (s). The compound can be detected but not isolated because of its ability:
BrF3 + Br2 → 3 BrF
BrF5 + 2 Br2 → 5 BrF
Br2(l) + F2(g) → 2 BrF(g)
Properties
- Chemical formula: BrF
- Molar mass: 98.903 g/mol
- Density: 4.403 g/L
- Melting point: −33 °C (−27 °F; 240 K)
- Boiling point 20 °C (68 °F; 293 K) (decomposes)
The above Bromine monofluoride chemical formula is based on the molecular formula, which indicates the numbers of each type of atom in a molecule without structural information, as opposed to the empirical formula, which provides the numerical proportions of atoms of each type.
The above chemical formula serves as the foundation for stoichiometry in chemical equations, which is the calculation of the relative quantities of reactants and products in chemical reactions. The law of conservation of mass states that in a chemical reaction, the quantity of each element given in the chemical formula does not change. As a result, based on the chemical formula, each side of the chemical equation must represent the same quantity of any particular element.
Structure
Bromine monofluoride’s 2D chemical structure image is also known as skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Bromine monofluoride are implied to be located at the corner(s), and hydrogen atoms attached to carbon atoms are not indicated – each carbon atom is assumed to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
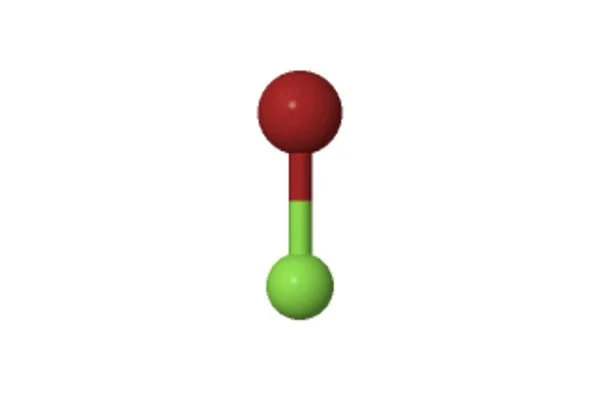
The 3D chemical structure image of Bromine monofluoride is based on the ball-and-stick model, which displays both the three-dimensional position of the atoms and the bonds between them. The radius of the spheres is therefore smaller than the rod lengths in order to provide a clearer view of the atoms and bonds throughout the chemical structure model of Bromine monofluoride.
Preparation
BrF can be prepared by the reaction of Br2 and F2 in various solvents below –100 °C. It is usually generated in the presence of cesium fluoride.
Bromine monofluoride decomposes at normal temperature through dismutation to bromine trifluoride, bromine pentafluoride, and free bromine.