Bromine trifluoride has the formula BrF3 and is an interhalogen compound. It is a colorless to yellow fuming liquid with a strong odor. It is a straw-colored liquid with a pungent odor that decomposes violently when it comes into contact with water or organic compounds. It is a strong fluorinating agent as well as an ionizing inorganic solvent. It is well known for its use as a powerful fluorinating agent.
It is used in the processing and reprocessing of nuclear fuel to produce uranium hexafluoride (UF6). This compound’s commercial applications are extremely limited. It is used as a fluoride solvent.
Properties
Bromine trifluoride is a colorless to gray-yellow fuming liquid that is noncombustible. It has a strong odor and is straw-colored. It dissolves in sulfuric acid but explodes when it comes into contact with water or organic compounds.
- Chemical formula: BrF3
- Molar mass: 136.90 g/mol
- Appearance: straw-coloured liquid hygroscopic
- Odor: Choking, pungent
- Density: 2.803 g/cm3
- Melting point: 8.77 °C (47.79 °F; 281.92 K)
- Boiling point: 125.72 °C (258.30 °F; 398.87 K)
- Solubility in water: Reacts with water
Preparation
Fluorination of bromine at 80°C can be used to produce bromine trifluoride. Diluting the halogen mixtures in nitrogen or an inert gas is an option. Paul Lebeau created this compound for the first time in 1906 by reacting bromine with fluorine at 20°C. The following equation describes the reaction:
Br2 + 3F2 → 2BrF3
Another way of producing Bromine trifluoride is to simultaneously reduce and oxidize Bromine monofluoride. This reaction produces Bromine trifluoride and bromine.
3BrF → BrF3 + Br2
Synthesis
Bromine trifluoride was first described by Paul Lebeau in 1906, who obtained the material by the reaction of bromine with fluorine at 20 °C:
Br2 + 3 F2 → 2 BrF3
The disproportionation of bromine monofluoride also gives bromine trifluoride:
3 BrF → BrF3 + Br2
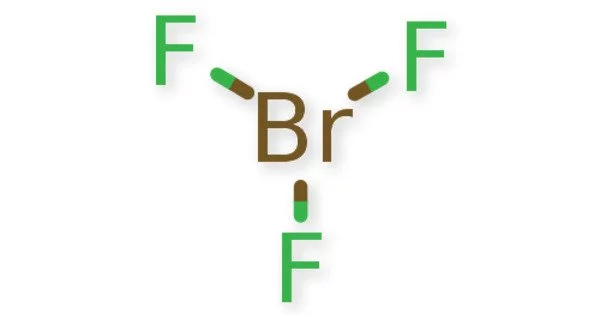
Structure
Like ClF3 and IF3, the BrF3 molecule is T-shaped and planar. In the VSEPR formalism, the bromine center is assigned two electron pairs. The distance from the bromine each axial fluorine is 1.81 Å and to the equatorial fluorine is 1.72 Å. The angle between an axial fluorine and the equatorial fluorine is slightly smaller than 90° — the 86.2° angle observed is due to the repulsion generated by the electron pairs being greater than that of the Br-F bonds.
Uses
Bromine trifluoride is a fluorinating agent and a strong ionizing inorganic solvent. It is also used in the production of uranium hexafluoride (UF6) during the processing and reprocessing of nuclear fuel.
Health Risk
Bromine trifluoride vapors are extremely irritating to the eyes, skin, and mucous membranes. When the liquid comes into contact with the skin, it can cause severe burns. This compound’s toxicity data are not available.
Inhalation causes severe upper respiratory system irritation. Contact with liquid or vapor causes severe eye burns, ulcers, and blindness. Contact with the skin results in severe burns. Ingestion results in severe mucous membrane burns.