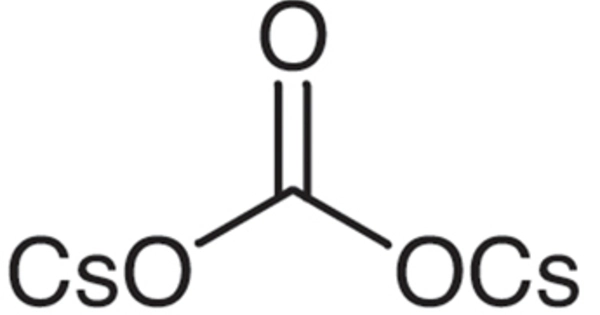
Cesium carbonate is an inorganic compound. Under normal temperature and pressure, it is a white solid. It is easily soluble in water and quickly absorbs moisture when exposed to air. It is a white crystalline solid compound. It is used in the manufacture of special optical glasses, petroleum catalytic additives, special ceramics, and the sulfuric acid industry.
Caesium carbonate is highly soluble in polar solvents such as water, alcohol, and DMF. It is more soluble in organic solvents than other carbonates such as potassium and sodium carbonates, but it remains insoluble in other organic solvents such as toluene, p-xylene, and chlorobenzene. This compound serves as a base in organic synthesis. It also appears to have applications in energy conversion.
Properties
- Chemical formula: Cs2CO3
- Molar mass: 325.82 g/mol
- Appearance: white powder
- Density: 4.072 g/cm3
- Melting point: 610 °C (1,130 °F; 883 K) (decomposes)
- Solubility in water: 2605 g/L (15 °C)
- Solubility in ethanol: 110 g/L
- Solubility in dimethylformamide: 119.6 g/L
- Solubility in dimethyl sulfoxide: 361.7 g/L
- Solubility in sulfolane: 394.2 g/L
- Solubility in methylpyrrolidone: 723.3 g/L
- Flash point: Non-flammable

Preparation
Caesium carbonate can be prepared by the thermal decomposition of caesium oxalate. Upon heating, caesium oxalate is converted to caesium carbonate with the emission of carbon monoxide.
Cs2C2O4 → Cs2CO3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.
2 CsOH + CO2 → Cs2CO3 + H2O
Cesium carbonate aqueous solution is strongly alkaline and can react with acid to produce corresponding cesium salt and water, and release carbon dioxide.
Chemical reactions
Caesium carbonate is very important for the N-alkylation of compounds such as sulfonamides, amines, β-lactams, indoles, heterocyclic compounds, N-substituted aromatic imides, phthalimides, and several similar other compounds. Research on these compounds has focused on their synthesis and biological activity.
In the presence of sodium tetrachloroaurate (NaAuCl4), caesium carbonate is a very efficient mechanism for aerobic oxidation of different kinds of alcohols into ketones and aldehydes at room temperature without additional polymeric compounds. There is no acid formation produced when primary alcohols are used.
Application
Cesium carbonate is a common cesium salt that is easy to transform and can be used as a precursor to other cesium salts. It is commonly used as a precursor to other cesium compounds. In sensitive organic reactions, it acts as a base.
Caesium carbonate can also be used in the reactions of Suzuki, Heck, and Sonogashira. Caesium carbonate produces carbonylation of alcohols and carbamination of amines more efficiently than some of the previous mechanisms. When a balanced strong base is required for sensitive synthesis, caesium carbonate can be used.