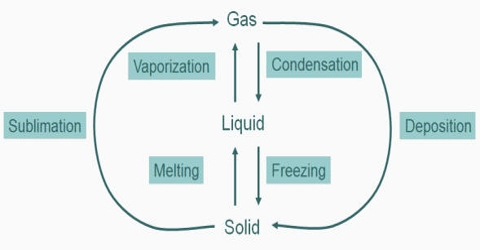
Change of State: Gas-liquid Transition
The kinetic theory of matter suggests that when a gas is subjected to high pressure the molecules will be brought closer to one another enabling them to exert attractive forces on each other. When the temperature is decreased the kinetic energy of the molecules would decrease so that the molecules would be sluggish and will not have enough energy to get away from each other alter collisions. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Every element and substance can transition from one phase to another at a specific combination of temperature and pressure. Either one or both of the operations of increasing pressure and decreasing temperature would, therefore, bring about liquefaction of gases.
In general, there is only one liquid phase of a material. However, there are two forms of liquid helium, each have some unique properties. Thus, the two forms are different (liquid) phases of helium. At a definite temperature and pressure, the two phases co-exist.
So far, all gases behave alike as do mixtures of gases. Thus, a gas is usually considered as a phase. Long before the kinetic theory of matter was accepted scientists were able to liquefy most gases by application of pressure or by decrease of temperature or both.
Some gases like N2, H2, He etc. escaped attempts at liquefaction even at the very low temperatures that could he attained at the time. These gases were called permanent gases.