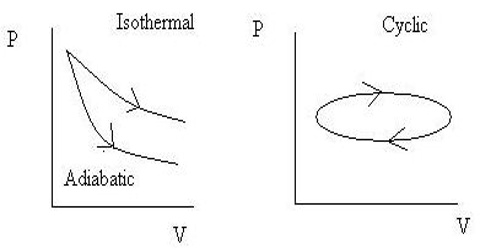
The cyclic process
A process in which a system goes from an initial state to a final state and returns back to the initial state is called a cyclic process. The net energy change in a cyclic process is zero. That means, in a cyclic process, the system starts and returns to the same thermodynamic state.
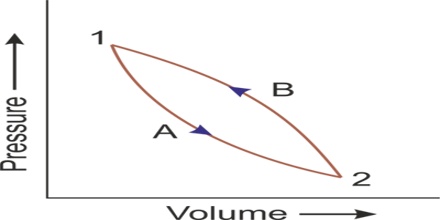
Let consider that a system changes from state A to state B, and then back to state A again. For the change from A to B, ∆U1 = UB – UA. And for the change from B to A, ∆U2 = UA – UB.
The total energy change,
∆Utotal = ∆U1 + ∆U2 = (UB – UA) + (UA – UB) = 0
For a cyclic process, ∆H must also be zero as H is a state function.