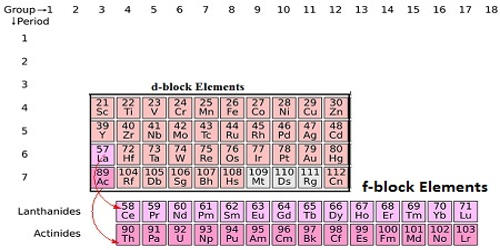
Elements whose f orbital getting filled up by electrons are called f block elements. The elements in which the extra electron enters ( n- 2 )f orbitals are called f-block elements. These elements are also called inner transition elements because they form a transition series within the transition elements. The f-block elements are also known as rare earth elements. These elements have electrons, (1 to 14) in the f orbital, (0 to 1) in the d orbital of the penultimate energy level and in the outermost’s orbital. These are divided into two series.
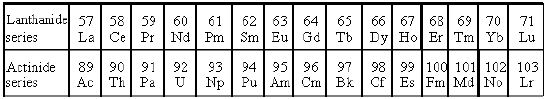
i) The Lanthanide series (4f-block elements) – In Lanthanides the differentiating electron enters 4f orbital. These are Cerium to Lutetium. The name lanthanides are because they come immediately after lanthanum.
ii) The Actinide series (5f- block elements) – In actinides, the differentiating electron enters 5f orbitals. These are thorium to lawrencium. These elements come immediately after actinium.
The position of f block elements in the periodic table is explained above. Lanthanides constitute the first inner transition series while actinides constitute the second inner transition series in chemical science. The electronic configuration of lanthanum and actinium has done filling electron in deep-seated 4f and 5f orbital with the increasing atomic number of the chemical element.
There are two series in the f block corresponding to the filling up of 4f and 5f orbitals. The elements are 4f series of Ce to Lu and 5f series of Th to Lw. There are 14 elements filling up the ‘f’ orbital in each series. The row beginning with Lanthanum is the row containing all the lanthanides whereas the row beginning with Actinium is the row that contains all the actinides. The differentiating electron in transition elements may enter either 4f or 5f orbitals based upon which they are differentiated into lanthanides and actinides.
Classification of F Block Elements
- The first series of elements are called lanthanides and include elements with atomic numbers beginning from 57 and ending at 71. These elements are non-radioactive (except for promethium, which is radioactive).
- The second series of elements are called actinides and include elements with atomic numbers beginning from 89 and ending at 103. These elements generally have a radioactive nature.
- The f–block elements of the periodic table consists of those elements whose atoms or ions have valence electrons in f–orbitals of the anti–penultimate shell.
Properties of F block Elements
- Have electrons added to the ‘f’ sub-orbitals of (n-2) level
- Are placed between (n-1)d and ns block elements in the periodic table.
- Properties are similar to d-block elements.
- f –block elements are also called inner transition elements. In these, the last electron enters penultimate.e.(n – 2) f orbital.