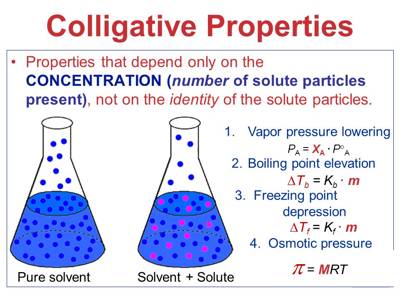
Colligative Properties
All molecular colloids in solution exhibit colligative properties. Since most of these colloids have high molecular masses the depression of freezing point, elevation of boiling point or lowering of vapor pressure is so small that these methods fail. In chemistry, colligative properties are properties of solutions that depend on the ratio of the number of solute particles to the number of solvent molecules in a solution, and not on the nature of the chemical species present. They can at best be used up to the molecular mass of a few thousand. The osmotic pressure, however, is quite marked for even high molecular mass polymers and this method finds wide use for the determination of molecular masses of a variety of polymers like proteins, cellulose and its derivatives, rubber, and a host of man-made polymers.
Modified osmotic pressure equation is used and the molecular mass is calculated from the intercept of a linear plot of π/C vs C. The extrapolation of the straight line is carried to zero concentration of the solution [π is the measured osmotic pressure and C is the concentration expressed in gL-1 solution].
Colligative properties are mostly studied for dilute solutions, whose behavior may often be approximated as that of an ideal solution. For osmotic pressure measurements, the colloid must be completely free from any electrolyte or any low molecular mass non-polymeric material to obtain any reliable value. It depends only on the number of dissolved particles in solution and not on their identity. Non-colligative properties depend on the identity of the dissolved species and the solvent.