Lyophilic colloids are liquid loving colloids (Lyo means solvent and philic means loving). Example: Sols of organic substances like gelatin, gum, starch, and proteins. Lyophobic colloids are liquid hating colloids (Lyo means solvent and phobic means hating). Example: Sols of inorganic substances like Arsenic (As2S3), Iron (Fe(OH)3) and Platinum.
Difference between Lyophobic Colloid and Lyophilic Colloid –
Lyophobic colloid: It forms a strong interaction between the dispersed phase and dispersion medium.
- Stability: Lyophobic colloids are thermodynamically unstable. These are unstable and hence require traces of electrolyte for stabilization.
- The only low concentration of the colloid is stable (usually 0.1-1%).
- Dialysis is to be done by rigid control of electrolytes; complete removal of electrolytes breaks the colloid.
- Interaction: There is less or no attraction between colloids and the liquid.
- Viscosity: Lyophobic colloids have the same viscosity as the solvent. It is about the same as that of the dispersion medium.
- Very sensitive to electrolyte concentration and coagulation is very easy.
- After phase separation by coagulation, re-dispersion of the coagulum is difficult and needs special treatment.
- The coagulation consists of discrete aggregated panicles.
- Exhibits marked light scattering and strong Tyndall effect.
- Most of the colloids are visible under high power microscope or ultramicroscope.
- Surface tension very close or similar to that of the dispersion medium.
- Viscosity is only slightly higher than that of the medium.
- Exhibits no solution properties, such as the colligative properties. The colloid is a two-phase mixture.
- Refractive index of the colloid does not follow any definite pattern.
- Water as the Solvent: When water is taken as the solvent, lyophobic colloids are known as hydrophobic colloids.
- Reversibility: Lyophobic Colloids: Precipitation in the lyophobic sol is an irreversible process. Once the particles are separated, they cannot be incorporated back into the sols by means of simple remixing. Thus, they are called irreversible.
- Colligative property: They have high osmotic pressure, less depression in freezing point, less elevation in boiling point and less lowering of vapor pressure.
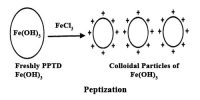
- Preparation: A lyophobic sol can be formed from special techniques such as mechanical agitation.
Examples: Metals such as platinum, gold, etc, metallic sulfides and hydroxides, sulfur, etc. are some examples.
Lyophilic colloid: It forms little or no interaction between the dispersed phase and dispersion medium.
- Stability: Lyophilic colloids are thermodynamically stable. These are stable and are self – stabilized.
- Relatively higher concentration can be attained without loss of stability.
- Relatively stable to dialysis and often dialysis is not necessary.
- Interaction: There is a strong attraction force between colloids and the liquid.
- Viscosity: Lyophilic colloids are highly viscous. It is much higher than that of the dispersion medium.
- Much less sensitive to electrolyte concentration; separation may occur at high electrolyte concentration.
- Re-dispersion of the separated phase is quite easy.
- Coagulation is often followed by ‘gel’ formation.
- Light scatting is low and little or no Tyndall effect.
- Most of the colloids are invisible even under ultramicroscope.
- Surface tension is usually lower than that of the dispersion medium.
- Viscosity is markedly increased.
- Exhibits properties of the solution, such as colligative properties; only the molecules are of colloidal dimension.
- Refractive index in most cases follows a definite pattern like that of true solutions.
- Water as the Solvent: When water is taken as the solvent, lyophilic colloids are known as hydrophilic colloids.
- Reversibility: Precipitation in the lyophilic sol is a reversible process. If the two phases are separated by using any separation technique, the sol can be recreated simply by mixing the phases. Thus, they are called reversible.
- Colligative property: They have relatively high osmotic pressure, depression in freezing point and high lowering in vapor pressure.
- Preparation: A lyophilic sol can be prepared by the direct addition of the dispersion phase (colloids) into dispersion medium (liquid).
Examples: Starch, gums, proteins, soaps, and metasilicic acids are some examples.
Colloids can be either lyophilic or lyophobic. Principally, lyophilic colloids are solvent loving particles, and lyophobic colloids are solvent hating particles. The major dissimilarity between lyophilic colloids and lyophobic colloids is that lyophilic colloids are thermodynamically constant whereas lyophobic colloids are unstable.